In the past decade, emerging therapeutic modalities like cell and gene therapies have opened up new treatment options for previously intractable diseases, changing the industry needs for primary tissues. While autologous therapies—which are created using a patient’s own cells—still dominate the field, many cell and gene developers are investing in allogeneic therapies that can be created using donor-sourced cells. Many advantages including simplified logistics, reduced cost and the ability to manufacture more consistent and higher quality products have led to this shift. Donor variability, scalability, regulatory compliance, and supply chain integrity are key challenges that a supplier must be able to accommodate in order to effectively serve the industry.
THE DONOR SOURCING CHALLENGE
In the manufacture of allogeneic cell therapies, upstream operations begin with isolation of lymphocytes from qualified donors via leukapheresis. These lymphocytes are the starting material that support all the downstream unit operations for cell and gene therapies such as gene editing, cell expansion, purification, formulation and cryopreservation. While AllCells’ standardized Good Manufacturing Practice (GMP) donor pool works for many clients, the diversity of cell and gene therapy programs often requires specific donor attributes because of the disease target or technology used. Thus, donor sourcing is the biggest upstream challenge that developers face. It can be difficult to find donors in the population who have the desired and possibly rare attributes needed for a given program, as well as ensuring that volume, quality and regulatory needs are met throughout a product’s lifecycle as it progresses from the bench to the clinic and eventually to market approval.
The condensed timelines faced by cell and gene therapy developers means that generalized, mass recruitment efforts to source donors fall short of meeting project-specific goals. Recognizing that a new approach was needed, LeukoLab—AllCells donor-facing brand—established a Donor Management System (DMS). The DMS is a proprietary system built by seasoned experts to meet the cell and gene therapy industry’s demand for unique donor qualifications.
DONOR MANAGEMENT SYSTEM (DMS)
The DMS was designed to provide a better way to target donors with the desired attributes to meet researchers’ specific program requirements and build upon the relationships that LeukoLab curates with its individual donors. Researchers are supported throughout their product lifecycle by a multi-departmental team and proprietary DMS software to navigate donor requirements from inception to commercialization based on timeline, risk and cost considerations. Whether a project has specific donor criteria, screening needs, collection cadence requirements or timeline restrictions, the Donor Management team can create customized solutions to ensure a high conversion from donor identification to successful collections. Years of experience in donor recruitment allows the Donor Management team to know how many donors must enter the screening funnel to achieve the number of qualified donors needed to fulfill the demand to meet the end goal (i.e., based on the desired donor attribute and its frequency in the population) and the best strategies to attract those potential donors with targeted recruitment campaigns. AllCells’ Donor Management Services comprise four key areas focused on donor recruitment, screening, monitoring, and retention.

The potential donor pool is ever-changing as individuals move in and out of availability. The Donor Management team is responsible for the movement of donors through the workflow to ensure that they are appropriately qualified and allocated based on the identified needs. After donors are recruited, they undergo extensive viral and health screening protocols to qualify for research or clinical-grade donations. Donors can also be subject to client-specific testing requirements as part of their qualification. Donors are then allocated into different donation pools based on standard or more stringent project specifications (i.e., dedicated donor pools). All donors are IRB-consented and collections are performed at one of AllCells’ FDA-registered collection facilities, which operate under a robust quality system. AllCells’ GMP products undergo additional, extensive quality review prior to batch release to ensure high quality standards and compliance with regulatory requirements needed for clinical use. For those donors reserved in dedicated donor pools, the Donor Management team engages in active monitoring and engagement to foster reliability and recallability, which minimizes donor variability and ensures supply chain continuity.
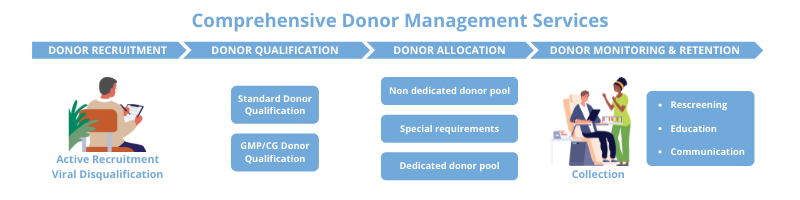
The goal of a good supplier is to use their industry expertise to support developers by de-risking cell and gene workflows that involve donor-sourced material in the face of a dynamic and diverse population. Open communication, know-how and comprehensive project management provide the tools necessary to navigate through unique donor and regulatory requirements for researchers’ programs. From determining project feasibility to providing guidance on donor criteria establishment and more, AllCells takes a consultative, holistic approach to anticipate and meet the needs of the industry now and in the future. Learn more about AllCells’ Donor Management Services.




