
AllCells® GMP Fresh Bone Marrow
Discovery AllCells® Clinical Grade Fresh Whole Bone Marrow is a rich source of adult stem cells such as CD34+ hematopoietic stem and progenitor cells. These cells are capable of differentiating into all of the mature blood cell types. Mesenchymal stromal/stem cells are also found in bone marrow and have been shown to have therapeutic potential in several cardiovascular, autoimmune, and immune-based diseases. A certificate of analysis and a comprehensive summary of record (SoR) documents are provided with every order. GMP Fresh Bone Marrow is available through consultation with an AllCells Made-to-Order representative.
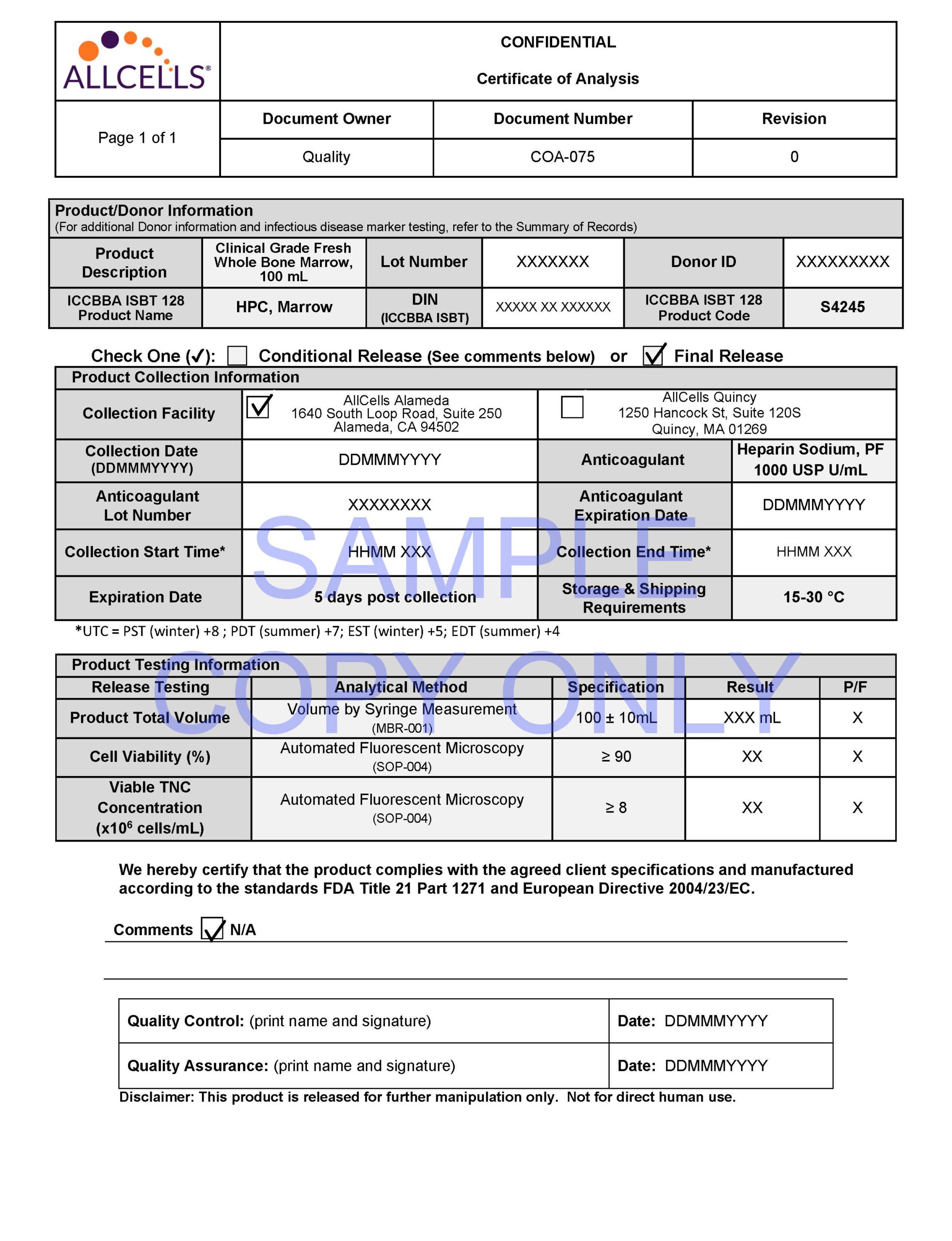
AllCells® GMP Bone Marrow is a rich source of CD34+ cells, mesenchymal stem cells (MSCs) and other leukocytes. Our products are quality controlled for cell concentration and viability using a validated AO/PI protocol. Sterility is optionally included and can be tested in accordance with USP<71> guidelines using compendial testing.
- Catalog Number: CG, BM, FR, 1ea (100mL)
- Anti-Coagulant: Sodium Heparin or ACD-A
- Volume: 100 ± 10mL
- Viability: NLT 90%
- TNC Concentration: NLT 8×106 cells/mL
- Optional Sterility Test USP <71>: No Growth
- Shipping Condition: 15 to 30°C in validated AeroSafe shipping container
- Documentation: Certificate of Analysis, Summary of Records
At AllCells, we believe that your success is our success. We believe that sharing our knowledge and expertise is more than a business practice, it’s a responsibility. And we believe that collaboration leads to better outcomes. Below is a collection of relevant links to Bone Marrow resouces including blog posts, webinars and useful protocols to help you become more efficient and more effective. Have technical questions about Bone Marrow products? Our Customer Success Team is standing by.
WEBINARS
BLOG POSTS
- Navigating the complex world of regulations: How AllCells is changing cell therapy and protecting against product-ending events
- The Key to Developing Good Manufacturing Practice (GMP) Biomaterials for Cell and Gene Therapy Development
- The Therapeutic Potential of HSC-based Gene Therapies
- Why You Should Start Clinical Grade Cell Supply Discussions Early in Cell and Gene Therapy Development
- What You Need to Know About Regenerative Medicine in 2022: Industry and Tech Trends
- A Donor-Centric Approach to Cell and Gene Therapy
PROTOCOLS

AllCells® GMP Fresh Bone Marrow Difference
- Seamlessly transition from process development to manufacturing with RUO Fresh Bone Marrow and GMP Fresh Bone Marrow
- Choose from two validated anti-coagulant options to meet program specific requirements
- Process without delays with 99% deliverability rate of GMP products
