Quality System
Discovery Life Sciences is renowned for providing customers with products of unparalleled quality, viability, and purity. Over the years, we have developed and optimized protocols to ensure that all of our AllCells® products and services consistently meet these high standards.

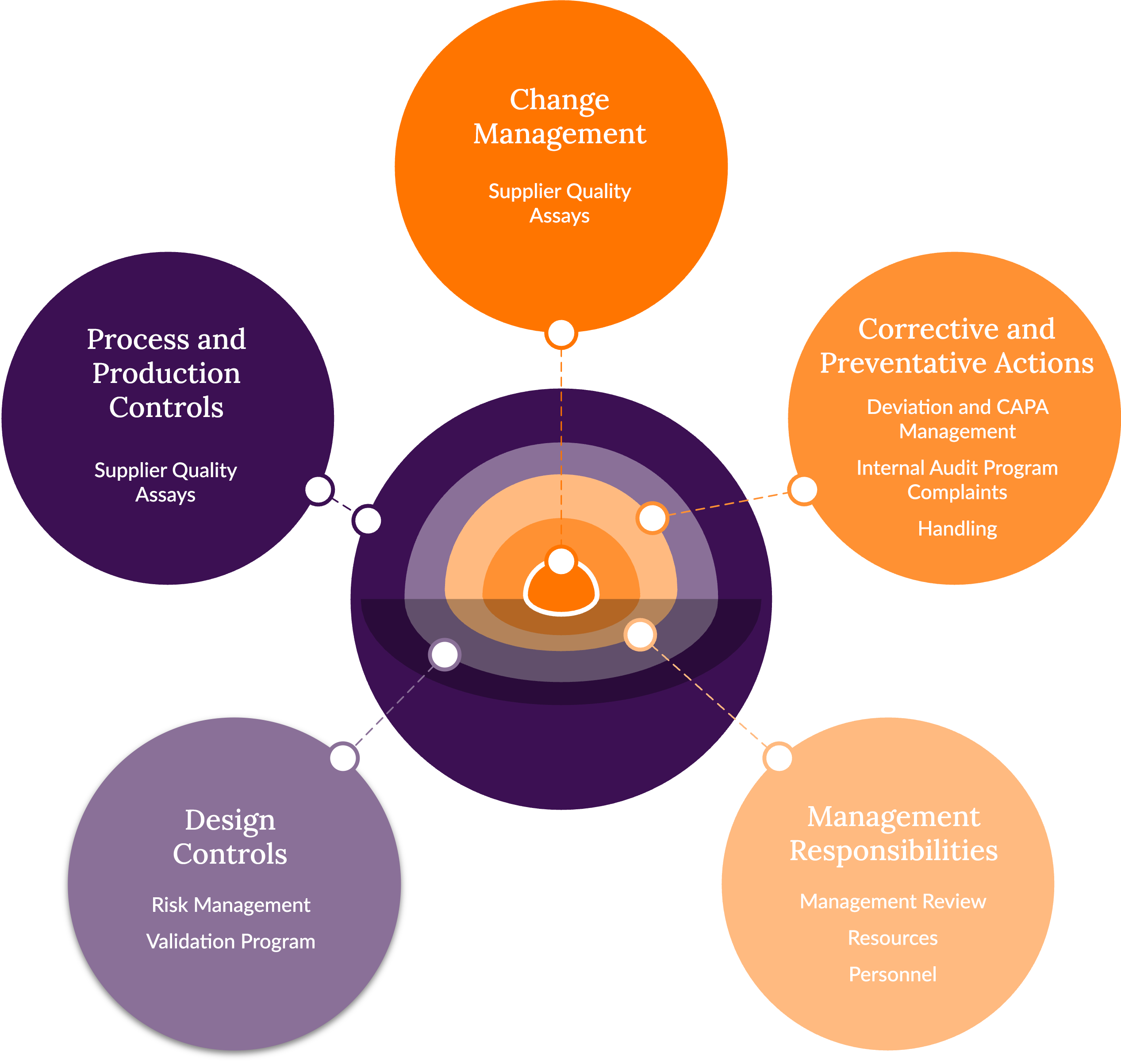
Discovery's Quality System Overview
The structure of Discovery’s Quality Management System (QMS) is based on the models outlined in ICH Q10, “Pharmaceutical Quality System” and follows and fulfills the guidelines that has been developed by the FDA:
- FDA Code of Federal Regulations (CFR) 21 Part 1271 – Human Cells, Tissues, and Cellular and Tissue-based Products.
- FDA CFR 21 Part 630 – Requirements for Blood and Blood Components intended for Transfusion or Further Manufacturing Use
Framework
- Based on the predicate requirements of FDA’s Title 21, Part 1271 AllCells’ products are not considered HCT/Ps, but as precursors to HCT/Ps produced by the customer. Products have a label claim of “For manufacturing use only. Not for direct human use.” As such, products are manufactured in accordance with GMP and FDA Title 21 Part 1271 Subparts A-C and provisions of Subpart D deemed applicable by Discovery under Section 1271.150 (c) and good industry practice.
- Products are also manufactured in accordance with European Directive 2004/23/EC, European Directive 2006/17/EC, where Discovery is defined as the Procurement Organization and Customer is defined as the Tissue Establishment.
- Our products (BM and LP) are considered as peripheral blood stem cells as per European Directive 2004/23/EC, European Directive 2001/83/EC
Registrations and Licenses
- FDA registration #3010226283
- Tissue Bank License by State of California Department of Public Health. Tissue Bank ID#: CTB 00080812
- License for the Production of Biologics by State of California Department of Public Health. Blood Bank ID# 9732
- Certificate of Compliance by Clinical Laboratory Improvement Amendments. CLIA ID: 05D2069499 (Alameda)
- Certificate of Compliance by Clinical Laboratory Improvement Amendments. CLIA ID: 22D2126670 (Quincy)
- Medical Director and Independent IRB-approved donor consent program
