The Therapeutic Potential of HSC-based Gene Therapies

Hematopoietic stem cells (HSCs) have immense therapeutic potential because of their ability for both self-renewal and multi-lineage differentiation to maintain hematopoietic homeostasis. This rare population of cells are characterized by the presence of the cell surface marker CD34, which is useful for their identification and isolation from the bone marrow and peripheral blood.
In the clinic, allogeneic (donor-derived) HSC transplantation (HSCT) is used routinely to treat a variety of hematological and immunological diseases. While HSCT can be curative, effectively correcting and reconstituting the entire hematopoietic system, it can be a challenge to find well-matched donors. Given their unique properties, the genetic modification of autologous (patient-derived) HSCs is an attractive therapeutic alternative for patients who lack a compatible donor. HSC-based gene therapy is an actively expanding area of therapeutic interest where several genetic engineering platforms are under clinical development.
Genetic Engineering Strategies
Most current genetic engineering of HSC falls into two main categories:
- Gene replacement for loss-of-function diseases mediated by viral vectors
- Targeted site-specific gene editing using programmable endonucleases for gene correction/addition
Lentiviral vectors are attractive for clinical applications because they can effectively transduce non-proliferating or slowly proliferating cells, such as CD34 + HSCs. However, the possibility of insertional mutagenesis and modified transgene expression pattern could pose safety risks to patients.
Targeted gene editing allows for site-specific genome modification that can replace a disease-causing gene, inactivate a disease-causing gene, or introduce a new or modified gene into the body to help treat a disease without the inherent risks posed by random gene insertion. Advances in engineered nucleases such as zinc finger nucleases (ZFNs), transcription activator-like effector nucleases (TALENs), and CRISPR/Cas9 (Clustered regularly interspaced short palindromic repeats/CRISPR-associated protein 9) and more recently CRISPR/Cas-based epigenetic, base- and prime-editing systems have transformed clinical gene therapy approaches. While each programmable nuclease platform is at different stages of clinical development, RNA-guided CRISPR/Cas-based systems are becoming the tools of choice for gene therapies because of their ease of use, specificity, and targeting versatility1.
These engineered endonucleases induce DNA double-strand breaks (DSBs) at the target chromosomal site to trigger one of two endogenous cellular DNA repair pathways: nonhomologous end joining (NHEJ) or homology directed repair (HDR). HDR is the preferred mechanism for gene editing where a donor DNA template can be supplied and accurately inserted into the genome of HSCs during DSB repair.
Gene engineering strategies employed can be broadly divided into in vivo or ex vivo gene editing approaches depending on the target disease (Figure 1). With in vivo gene therapy, the therapeutic gene is administered directly to the patient. This is typically accomplished by systemic or local injection of viral vectors or newer delivery technologies like lipid nanoparticles that contain the genetic payload. Ex vivo gene therapy involves removing the target cells from the patient, modifying them, before they are returned to the patient’s body. Gene editing components are delivered to HSCs using physical (i.e., electroporation), chemical (i.e., lipofectamine) or viral vector systems (i.e., adeno-associated viral vectors (AAV)). Thanks to positive safety and efficacy data, a number of ex vivo HSC gene therapy programs are rapidly progressing to late-stage clinical trial evaluation in the US and abroad.
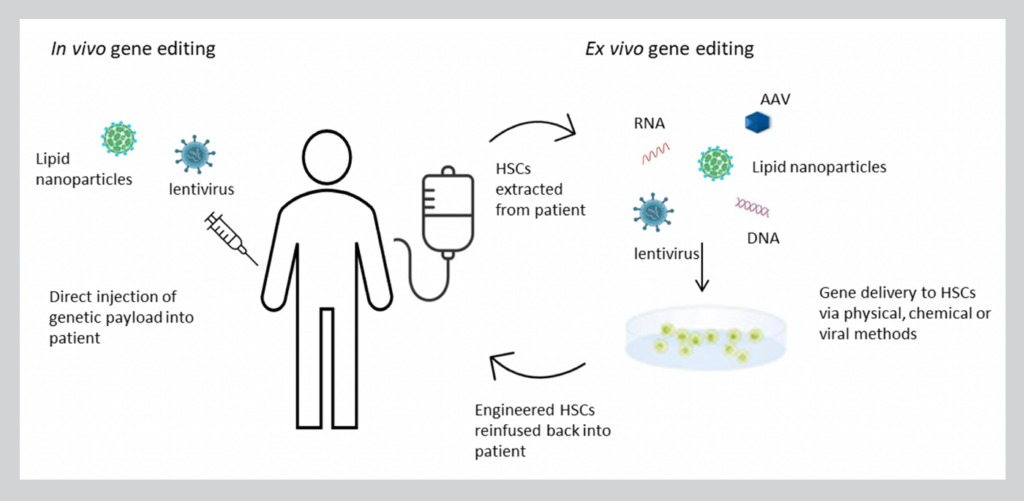
Figure 1. In vivo vs. ex vivo HSC gene editing strategies.
Tissue Sources of HSCs for Gene Therapy
HSCs can be isolated from bone marrow aspiration from the iliac crests under general anesthesia. Alternatively, HSCs can be obtained from mobilized peripheral blood (MPB) collected by leukapheresis. This procedure is well-tolerated and the ease of leukapheresis collection has made MPB the tissue source of choice of CD34+ cells for clinical applications.
Currently, there are three FDA-approved mobilization agents for clinical use that can effectively recruit HSCs from the BM niche into the bloodstream: granulocyte colony stimulating factor (G-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF) and the small molecule drug Plerixafor2. Evidence shows that the combination of G-CSF and Plerixafor results in enhanced mobilization of HSPCs with greater repopulating potential in donors compared to G-CSF or GM-CSF alone. G-CSF acts on the CXCR4/SDF-1α pathway to degrade proteins anchoring HSCs to the marrow stroma to release the CD34+ cells into the circulation. Plerixafor reversibly inhibits binding of SDF-1α to CXCR4 receptor to further increase mobilization. The mobilized peripheral blood is then harvested using specialized apheresis platforms, such as the industry-leading Spectra Optia® system (Terumo BCT), followed by isolation of CD34+ cells (i.e., positive immunomagnetic separation), genetic modification and expansion in the laboratory, prior to infusion into the patient.
Therapeutic Applications
HSC-based gene therapies are being developed by leading biopharma companies for a broad range of blood-specific diseases, such as primary immunodeficiencies (PIDs), hemoglobinopathies, congenital forms of cytopenia, and stem cell defects, in addition to metabolic diseases such as leukocyte adhesion deficiency-I, and rare disease indications like Gaucher and Hunter Syndrome3.
Primary Immunodeficiencies
Severe combined immunodeficiencies (SCIDs) were the first monogenic disorders for which HSC gene therapy has been successfully developed. In fact, Strimvelis, a lentiviral gene therapy for adenosine deaminase deficiency SCID (ADA-SCID), was approved in 2016 by the European Medicines Agency (EMA). ADA-SCID is caused by a mutation in the adenosine deaminase (ADA) enzyme gene. Engineered HSCs containing a functional copy of the ADA gene can restore function in vivo as a curative treatment for ADA-SCID. Follow-up studies indicate a majority of patients treated with the therapy retained robust immune function two to three years after receiving treatment with few adverse effects. Engineered HSCs are currently under investigation for several other PIDs including SCID-X1, Wiskott-Aldrich syndrome, and chronic granulomatous disease with promising results4.
Hemoglobinopathies
CRISPR Therapeutics is in phase 1/2 clinical trials for an ex vivo CRISPR-edited gene therapy CTX001 that it’s co-developing with Vertex. This is a potential one-time therapy for patients suffering from transfusion-dependent β-thalassemia (NCT05356195) and severe sickle cell disease (NCT05329649). Autologous HSCs are engineered to produce high levels of fetal hemoglobin (HbF) in red blood cells to alleviate (or eliminate) transfusion requirements for β-thalassemia patients and minimize or eliminate sickle crises in SCD patients.
Metabolic Diseases
Rocket Pharmaceuticals recently released interim data from phase 1/2 clinical trial (NCT03812263) of its lentiviral-mediated ex vivo gene therapy for pediatric patients suffering from severe Leukocyte Adhesion Deficiency-I (LAD-I) caused by mutations in the ITGB2 gene encoding for the beta-2 integrin component CD18. RP-L201 consists of patient-derived HSCs that have been genetically modified to contain a functional copy of the ITGB2 gene. The interim results of the trial at 3-24 months post RP-L201 infusion showed sustained stable CD18 expression with no serious therapy-related adverse events. Rocket Pharmaceuticals is also developing HSC gene therapies to treat Fanconi’s Anemia and Pyruvate Kinase Deficiency.
Rare Diseases
AVROBIO is evaluating an investigational ex vivo HSC gene therapy AVR-RD-02 in a phase 1/2 clinical trial (NCT04145037). The therapy aims to treat patients suffering from Gaucher Type 1 disease—a rare, inherited lysosomal disorder caused by a mutation in the GBA gene, which makes the enzyme glucocerebrosidase. Cells are unable to break down lipids resulting in harmful accumulation of lipids within tissues in the body that can cause systemic issues. AVROBIO is also developing HSC gene therapies for other lysosomal storage disorders like cystinosis, Hunter Syndrome, and Pompe.
The therapeutic applications highlighted here are autologous HSC gene therapies and while their clinical potential is undeniable, yet the merits of allogeneic donor cells are worth serious consideration. Engineering healthy donor cells to produce “off-the shelf” products could be used to treat multiple patients, improving accessibility and reducing the cost of treatment5. That said, allogeneic HSC gene therapy approaches still face serious hurdles that will need to be overcome similar to allogeneic CAR-T therapies. Most notably, life-threatening reactions can arise from patient rejection of the engineered donor cells (graft vs. host disease).
Because HSC-based gene therapy products are regulated by the FDA’s Center for Biologics Evaluation and Research (CBER), it is necessary to establish a comprehensive good manufacturing practice (GMP) process from beginning to end—from cell harvest, isolation to genetic modification and expansion to produce a suitable clinical-grade product. The quality of the engineered HSCs is impacted by donor-to-donor variability and is also dependent on the manufacturing environment where a robust and reproducible GMP manufacturing process is essential to ensure product consistency and safety2. GMP donor cells can be utilized to execute CMC (chemistry, manufacturing, and controls) activities including process development and process performance qualification (PPQ) to accelerate the development and commercialization of HSC gene therapies. In fact, leaders in the autologous field are already implementing GMP mobilized leukopaks to support the regulatory filings for current products in their pipeline. Regulators are more likely to give their stamp of approval when companies show they are committed to the quality and safety of their therapeutic with high-quality starting materials and have the GMP documentation to support that. This also helps set a precedent and provides a path forward for other similar programs in a company’s pipeline that can have long-lasting benefits.
GMP Mobilized Leukopaks
AllCells’ new GMP Mobilized Leukopaks are part of its expanding GMP product portfolio to facilitate the advancement of autologous and allogeneic HSC gene therapies from the lab to clinic. Access to quality controlled, scalable supply of clinical-grade donor CD34+ cells provides a consistent foundation to perform a wide variety of studies from proof-of-concept, IND-enabling studies, manufacturing process optimization/validation, and clinical validation across both autologous and allogeneic programs.
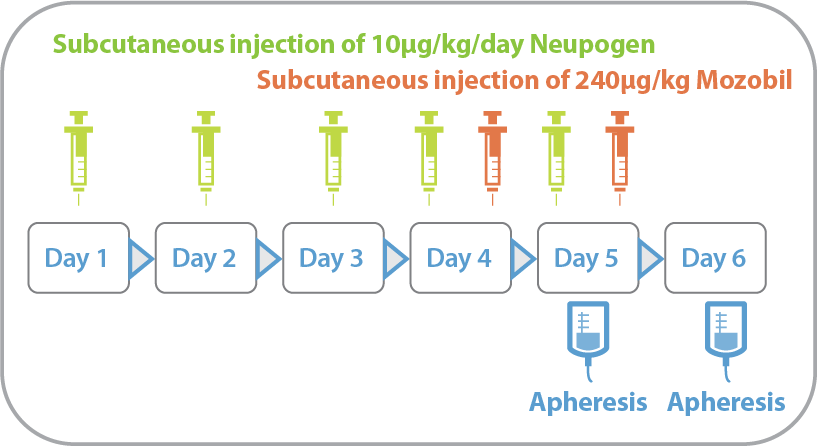
Figure 2. Mobilization Regimen H: Filgrastim (Neupogen) 10ug/kg/day x 5 days + Plerixafor (Mozobil) 240ug/kg/day x 2 days on day 4 and 5 (evenings), apheresis on day 5 and 6
Validated clinical donors who meet FDA 21 CFR 1271 requirements undergo a standardized RegH mobilization regimen (Figure 2) using FDA-approved drugs Neupogen (G-CSF) and Mozobil (Plerixafor), which effectively recruits CD34+ hematopoietic stem and progenitor cells from the bone marrow into peripheral blood. Subsequent leukapheresis using the Spectra Optia apheresis system at AllCells’ FDA-registered, IRB-approved, AABB-compliant donor centers results in high quality, high yield single donor CD34+ collections. Extensive quality testing and extended GMP documentation accompanies every batch, providing developers with the starting material consistency, scalability, and regulatory compliance needed to advance their therapeutic programs. AllCells’ donor management and GMP teams can work collaboratively with developers to find, manage, and collect from donors with program-specific attributes at the desired cadence.
The complement of the RUO and GMP Mobilized Leukopaks delivers a comprehensive portfolio of early-stage research and manufacturing solutions encompassing the entire product pipeline from preclinical R & D to commercialization.
Learn more about GMP Mobilized Leukopaks
References
- Klaver-Flores S, Zittersteijn HA, Canté-Barrett K, et al. Genomic Engineering in Human Hematopoietic Stem Cells: Hype or Hope?. Front Genome Ed. 2021;2:615619. Published 2021 Jan 22. doi:10.3389/fgeed.2020.615619
- Wang X, Rivière I. Genetic Engineering and Manufacturing of Hematopoietic Stem Cells. Mol Ther Methods Clin Dev. 2017;5:96-105. Published 2017 Mar 18. doi:10.1016/j.omtm.2017.03.003
- Staal FJT, Aiuti A, Cavazzana M. Autologous Stem-Cell-Based Gene Therapy for Inherited Disorders: State of the Art and Perspectives. Front Pediatr. 2019;7:443. Published 2019 Oct 31. doi:10.3389/fped.2019.00443
- Tucci F, Scaramuzza S, Aiuti A, Mortellaro A. Update on Clinical Ex Vivo Hematopoietic Stem Cell Gene Therapy for Inherited Monogenic Diseases. Mol Ther. 2021;29(2):489-504. doi:10.1016/j.ymthe.2020.11.020
- Morgan RA, Gray D, Lomova A, Kohn DB. Hematopoietic Stem Cell Gene Therapy: Progress and Lessons Learned. Cell Stem Cell. 2017;21(5):574-590. doi:10.1016/j.stem.2017.10.010