AllCells® GMP Products
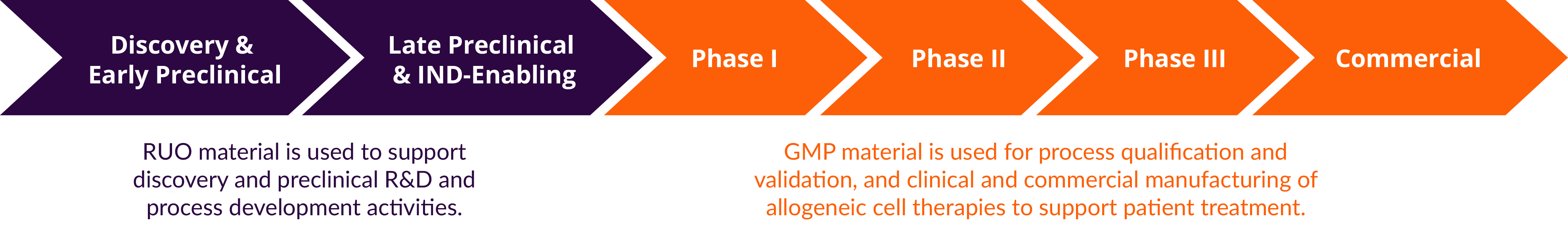
Discovery’s proven capabilities help clients navigate and streamline the complex journey from product development to commercialization. High quality research use only (RUO) products support drug discovery, preclinical testing, and process development, providing researchers confidence to progress therapies into the clinic. Discovery’s AllCells® GMP products ensure regulatory compliant starting cellular material for clinical and commercial manufacturing at scale.

Serving the Full Continuum of Cell and Gene Therapy
Partnering with Discovery for RUO and GMP products to span the entire workflow mitigates timeline and cost delays that could arise from having to revalidate source material and suppliers. This also provides the flexibility and agility to bilaterally transition between pre-clinical and clinical phases to address the dynamic nature of cell therapy product development.
GMP Cryopreserved Leukopak
Manufactured in Discovery’s GMP ISO 7 cleanroom as made-to-stock and custom options.
GMP Mobilized Leukopak
Mobilized, large-scale collections of CD34+ hematopoietic stem and progenitor cells for GMP manufacturing.
GMP FRESH LEUKOPAK
Our fresh leukopaks contain a high concentration of lymphocyte populations, T Cells, B Cells, NK Cells, and monocytes.
GMP BONE MARROW
Bone marrow is enriched in hematopoietic stem cells (HSC) and mesenchymal stem cells (MSC) capable of multi-lineage differentiation into a number of clinically relevant cell types.
Why Use AllCells® GMP Tissues and Cells
Reliable and Trusted GMP Supplier
With years of providing materials for GMP use and over 20 years in the industry, Discovery is your reliable and trusted supplier of high-quality starting material
Integrated Project Management
Our collaborative and dedicated team of scientists, quality, donor and project management experts will customize your project, pulling from extensive experience and donor data
Robust and Assured Supply Chain
Discovery’s highly reliable, recallable, and dedicated GMP eligible donor pool and geographically diverse facilities are designed for scalability
99% GMP Collections Deliverability
Mitigate the cost of failure with built-in risk reduction steps including infectious disease screening across various time points and parallel donors
FAQs
What is the difference between RUO and GMP?
Discovery maintains the same high quality material and optimized protocols for both RUO and GMP products. Since GMP products are intended for further manufacturing for clinical and commercial use, they must meet rigorous standards, which are applied at multiple levels:
| Parameter | GMP | RUO |
|---|---|---|
| Donor Assessment and Testing | Donors are subject to infectious disease marker testing in compliance with 21 CFR 1271, as well as those recommended by CDC and AABB. Donors also undergo a physical exam and must pass the donor health questionnaire. | Donors are subject to infectious disease marker testing for HIV, Hepatitis B, and Hepatitis C. Donors also undergo a physical exam. |
| Donor Consent (IRB Approved) | For research and commercial use. | For research use only |
| Operational SOPs | Validated SOPs and extensive batch records. | Validated SOPs/e-batch records |
| Regulatory Compliance | Products are manufactured in accordance with FDA Title 21 Part 1271 Subparts A-C and provisions of Subpart D deemed applicable by Discovery under Section 1271.150(c) and good industry practice. | For research use only |
| Quality | QMS-governed documentation e.g. batch records, equipment and product validation, stability studies, to support product for further manipulation. | E-documentation, product specifications based on process experience, certificate of analysis |
| Processing Facility | ISO 7 cleanroom with Grade A and B zones. | GMP-like collection and processing facilities |
| Packaging Protocol and Packaging | Packaging validated to ISTA standards including temperature logger. | Commercial grade |
What steps does Discovery take to maximize the success of GMP collections?
In addition to recruiting and maintaining reliable, recallable donors (see our donor difference), Discovery approaches GMP collections with risk mitigation steps including infectious disease screening across various time points, backup donors, and advising shipments under severe weather conditions. We regularly customize programs and advise on screening workflows, pulling from our extensive experience and donor data.
How does Discovery qualify donors as eligible for GMP collections?
Discovery’s GMP-eligible donors are reliable, recallable, and must test negative for infectious diseases as specified in 21 CFR Part 1271.
- Human immunodeficiency virus Type 1 antibodies and RNA
- Human immunodeficiency virus Type 2 antibodies and RNA
- Hepatitis B antibodies and DNA
- Hepatitis C antibodies and RNA
- Human T-lymphotropic Virus I/II antibodies
- Cytomegalovirus (CMV) antibodies
- West Nile virus RNA
- Trypanosoma cruzi parasite (Chagas Disease) antibodies
- Treponema pallidum (Syphilis) antibodies
Donors must undergo physical exams as well as pass the Donor Health Questionnaire to ensure that they do not have any other health conditions that would disqualify them from GMP collections. We will collaborate with you to meet additional program-specific and regional regulatory requirements as required.
Why should I use GMP starting materials early?
The quality of the starting cellular material has a direct impact on the quality of the final product. GMP products help establish a robust and reproducible manufacturing process to ensure product consistency and safety.
What if I need GMP products that are not listed in the AllCells® product portfolio?
We are continuously working to expand our GMP product offerings and are happy to discuss potential solutions. Please fill out a form on our website or contact your account representative detailing your GMP needs.
Donor Management Services
At Discovery, we understand that finding donors who meet regulatory criteria are just as critical to the success of the product as the specific program requirements themselves. Our GMP-eligible donors are reliable and highly characterized, including HLA and KIR typing, CMV status, blood type, demographics, and lifestyle characteristics.

Need More Information?
We realize the importance of finding the right partner for your research and want to make it as easy as possible for you to find the answers to all of your questions. Feel free to contact our Customer Support Team via our easy email form below.