Isolated human primary cells are able to retain the morphological and functional characteristics of their tissue of origin, which makes them invaluable tools in life sciences to advance our understanding of cellular biology and molecular mechanisms that are aiding researchers in the development of novel therapeutic approaches to treat human diseases. For instance, primary lymphocytes like T cells have been the focus of ground-breaking precision medicine strategies like chimeric antigen receptor (CAR)-T cell immunotherapies that have demonstrated unprecedented clinical successes. Unlike cell lines, which can be immortalized and be cultured long-term, primary cells have a finite lifespan and are difficult to maintain in cell culture after isolation. Extended culture can result in loss of pluripotency and/or functionality resulting from epigenetic changes and genetic drift that may alter their responses to stimuli compared to cells in vivo1. Therefore, most research and clinical programs rely on access to freshly collected cells from blood banks or commercial suppliers to execute their studies.
Leukopaks are peripheral blood leukapheresis products enriched in mononuclear cells (PBMCs) containing clinically relevant cell populations like T cells, B cells, NK cells, and monocytes, and are frequently used in the scientific community to address a variety of complex biological questions. Freshly collected Leukopaks have a relatively narrow window where their viability and functionality post-collection is retained but these attributes decline rapidly over time, which can negatively impact experimental results, costing time, money and resources. Cryopreservation of Leukopaks at the peak of viability and functionality preserves cell phenotypes and offer an excellent alternative with extended stability for timely and successful delivery of allogeneic cell-based therapies. Properly stored, they can be used weeks or months later with little decrease in the viability and potency of the cells, providing researchers with simplified logistics and timeline flexibility as well as mitigating the risks associated with donor scheduling delays, cancellations or courier-related issues. Cryostorage can also facilitate collaboration between organizations for the purposes of process design and optimization of downstream manufacturing2.
CRYOPRESERVATION PRESERVES IN VIVO FUNCTIONALITY
The ultimate goal of cryopreservation is to minimize damage to cells during the freezing process and subsequent storage, which can be difficult to accomplish if done incorrectly. Optimal cryopreservation depends on several key factors, including the cell type, cryoprotectant formulation, cooling rate of the cells and the equipment to maintain high recovery, viability and functionality upon thaw1-4. The main steps in cryopreservation are outlined in Figure 1.
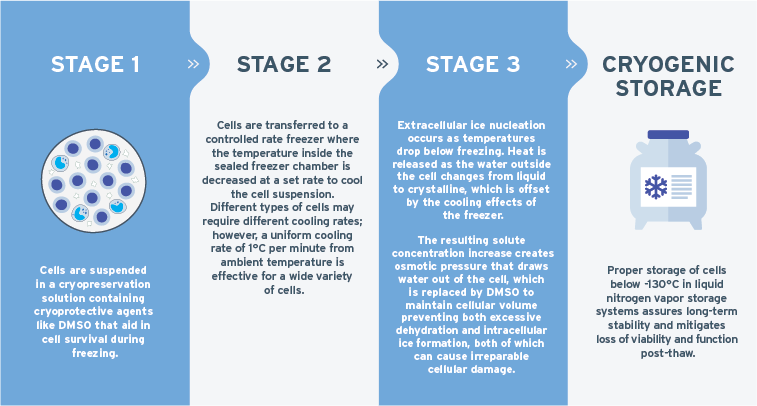
Dimethyl sulfoxide (Me2SO; commonly referred to as DMSO) has historically been the cryoprotectant of choice for most animal cell systems where the most commonly used concentration of DMSO is 10% v/v5. DMSO-containing CryoStor® is a preferred serum-free, animal component-free and defined cGMP formulation optimized for the cryopreservation of cells and biologics for research and clinical purposes6. Comparative studies have shown that PBMCs cryopreserved in Cryostor10 demonstrated superior post-thaw cell yield, viability, and recovery compared to 10% FBS (serum) formulations7.
Controlled rate freezer (CRF) units, which are actively cooled via liquid nitrogen or other mechanisms, as opposed to passive cooling devices (i.e., Mr. Frosty™), are preferred particularly for clinical usage owing to their cooling rate control and temperature monitoring for quality assurance purposes to meet regulatory guidelines. Buhl et al directly compared CRFand passive cooling rate for the cryopreservation of PBMCs and found CRF provided higher cell recovery, viability and maintenance of dendritic cell functionality post-thaw for their downstream immunotherapy protocol8. The cooling rate at which cells are cryopreserved has a big impact on its long-term viability and is highly dependent on the permeability of the plasma membrane of each cell type with regards to the kinetics of ice nucleation. When stored appropriately, the cells are preserved at ultra-low temperatures (-80 °C or -196 °C), which slows or completely stops biological activity2,6. Cryopreservation is a complex process and thus necessitates optimization of protocols and reagents to ensure high post-thaw viability and recovery since not all cells respond equally to a given cryopreservation protocol.
ALLCELLS GMP CRYOPRESERVED LEUKOPAKS
AllCells has developed an optimized cryopreservation protocol to provide GMP cryopreserved leukopaks that deliver high cell viability of important lymphocyte populations suitable for cell and gene therapy workflows from process development, optimization and large-scale manufacturing requirements.
Leukapheresis products are collected at FDA-registered, AABB-compliant collection facilities from qualified, healthy IRB-consented donors as per 21 CFR 1271 regulatory guidelines using the continuous flow Spectra Optia® Apheresis System directly into ACD-A anticoagulant. Fresh leukopaks are immediately processed in adjacent GMP labs to minimize the time between collection and cryopreservation, which is known to be a key determinant of post-thaw viability and recovery1. GMP leukopaks are cryopreserved using CryoStor10 and are processed within controlled environment rooms (Class A and B) using a proprietary Master Batch Record (MBR), which is optimized to maintain PBMC viability while mitigating granulocyte contamination. Granulocytes have a short half-life and do not survive cryopreservation processes. Upon thawing, the cells undergo lysis, releasing DNA and lysosomal enzymes that cause cell clumping and negatively impact post-thaw cell recovery9. There is also evidence that some factors released by the granulocytes can impact the post-thaw functionality of T cells, which can confound downstream protocols10. GMP cryopreserved leukopaks undergo extensive quality review prior to batch release to ensure the highest quality standards are met and in additional compliance with GMP requirements for downstream applications for cell-based products.
In direct comparisons between fresh and post-thaw cryopreserved leukopaks, no statistically significant differences were observed for the average % viability (Figure 2a) and viable cell counts (Figure 2b). Additionally, cryopreservation did not alter the proportion of mononuclear subpopulations assessed (Figure 2c; T cells, B cells, NK cells and monocytes).



Figure 2 a-c. Comparison between Fresh and Post-Thaw Cryopreserved Leukopaks. Average % viability, viable cell count and the distribution of MNC subpopulation were compared and no statistical difference was observed.
GMP cryopreserved leukopaks offer the benefits of a consistent supply of high-quality cellular materials with timeline flexibility that overcomes the risk of failure resulting from unexpected donor or logistics issues. To ensure end-to-end cold chain logistics are worry-free, AllCells ships GMP cryopreserved leukopaks using validated shipping containers (Aerosafe and CryoPort) provides a secured cryochain, eliminating temperature excursions that can cause product degradation during transit. Having a reliable and robust supply chain to meet the condensed timelines of cell and gene therapy workflows and adherence to GMP guidelines assures downstream manufacturing success and rapid movement through the product pipeline.
Learn more about GMP Cryopreserved Leukopaks.
REFERENCES
- Hunt CJ. Technical Considerations in the Freezing, Low-Temperature Storage and Thawing of Stem Cells for Cellular Therapies. Transfus Med Hemother. 2019;46(3):134-150. doi:10.1159/000497289
- Meneghel J, Kilbride P, Morris GJ. Cryopreservation as a Key Element in the Successful Delivery of Cell-Based Therapies-A Review. Front Med (Lausanne). 2020;7:592242. Published 2020 Nov 26. doi:10.3389/fmed.2020.592242
- Cytiva. Freezing Cellular Raw Materials For Cell Therapy Production. BioProcess Online. March 5, 2019. Accessed October 10, 2021. https://www.bioprocessonline.com/doc/freezing-cellular-raw-materials-for-cell-therapy-production-0001
- Fuller B, Gonzalez-Molina J, Erro E, Poirier A, Selden C, Awan M, Chalmers S, De Mendonca J. Applications and optimization of cryopreservation technologies to cellular therapeutics. Cell Gene Therapy Insights 2017; 3(5), 359-378. 10.18609/cgti.2017.038
- Awan M, Buriak I, Fleck R, et al. Dimethyl sulfoxide: a central player since the dawn of cryobiology, is efficacy balanced by toxicity?. Regen Med. 2020;15(3):1463-1491. doi:10.2217/rme-2019-0145
- BioProcess Online. BioLife Solutions CryoStor Cell Freeze Media Used In Mayo Clinic Safety And Feasibility Study Of Umbilical Cord Blood-Derived Cells For Pediatric Cardiac Regeneration. March 3, 2015. Accessed October 10, 2021. https://www.bioprocessonline.com/doc/biolife-solutions-cryostor-pediatric-cardiac-regeneration-0001
- Kofanova OA, Davis K, Glazer B, et al. Viable mononuclear cell stability study for implementation in a proficiency testing program: impact of shipment conditions. Biopreserv Biobank. 2014;12(3):206-216. doi:10.1089/bio.2013.0090
- Buhl T, Legler TJ, Rosenberger A, Schardt A, Schön MP, Haenssle HA. Controlled-rate freezer cryopreservation of highly concentrated peripheral blood mononuclear cells results in higher cell yields and superior autologous T-cell stimulation for dendritic cell-based immunotherapy. Cancer Immunol Immunother. 2012;61(11):2021-2031. doi:10.1007/s00262-012-1262-0
- Agashe C, Chiang D, Grishin A, et al. Impact of granulocyte contamination on PBMC integrity of shipped blood samples: Implications for multi-center studies monitoring regulatory T cells. J Immunol Methods. 2017;449:23-27. doi:10.1016/j.jim.2017.06.004
- McKenna KC, Beatty KM, Vicetti Miguel R, Bilonick RA. Delayed processing of blood increases the frequency of activated CD11b+ CD15+ granulocytes which inhibit T cell function. J Immunol Methods. 2009;341(1-2):68-75. doi:10.1016/j.jim.2008.10.019