Chimeric antigen receptor (CAR)-T cell therapy has transformed the treatment landscape for hematological malignancies such as relapsed/refractory acute B-cell lymphoblastic leukemia (ALL), diffuse large B-cell lymphoma (DLBCL), and multiple myeloma. Since 2017, six commercial CAR-T products have received FDA approval, with many more candidates advancing through the clinical pipeline1.
Despite its promise, the current manufacturing process to produce clinical-grade CAR-T cells remains complex, time-intensive, and highly expensive. This workflow begins with leukapheresis, followed by T cell isolation, genetic modification, and ex vivo CAR-T cell expansion (Figure 1). For allogeneic CAR-T cell therapies, non-mobilized leukopaks collected from healthy donors provide the starting material for T cell isolation. Variability in leukopak quality, however, can significantly impact manufacturing success and subsequently, therapeutic outcomes.
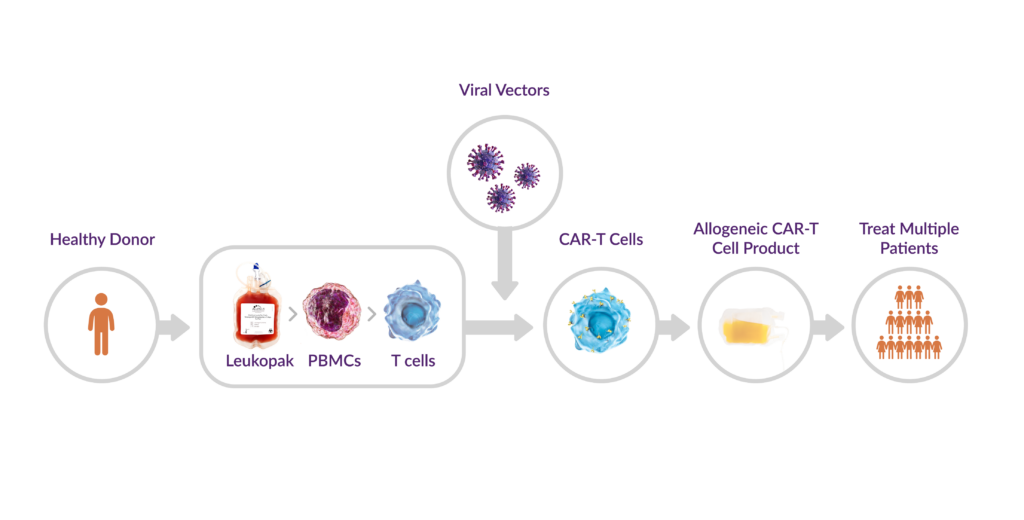
Figure 1. Allogeneic CAR-T cell workflow
Why Leukopak Quality Matters
Leukopak quality is critical to CAR-T therapy success—it can make the difference between whether a manufacturing run succeeds or fails and even if the CAR-T treatment will work2. Simply put, quality in leads to quality out.
During leukapheresis, peripheral blood mononuclear cells (PBMCs) are selectively isolated from other blood components, such as red blood cells, platelets, and plasma using a specialized device (i.e., Spectra Optia® Apheresis System). PBMCs are a heterogeneous mix of immune cells, including lymphocytes (T cells, B cells, natural killer (NK) cells), monocytes, and dendritic cells. However, the presence of contaminating cell populations in leukopaks, such as granulocytes, is a major issue in CAR-T cell therapy development.
High granulocyte levels in a leukopak can reduce ex vivo T cell expansion, a key step in CAR-T cell production, resulting in lower yields of viable target T cells, which can compromise downstream steps and diminish the likelihood of achieving therapeutic goals. An optimal leukapheresis product from high-quality donors is essential to the success of CAR T-cell therapy; therefore, understanding factors that may affect the quality or T-cell content is imperative2,3.
How Granulocyte Contamination Compromises T Cell Expansion
Granulocyte contamination and activation have multiple impacts on leukopak quality across CAR-T therapy development—from research and development (R&D) to clinical-grade manufacturing (GMP). High granulocyte levels can reduce the relative number of T cells in PBMC populations and inhibit T cell expansion4. Furthermore, contaminating granulocytes have been associated with cellular integrity loss in regulatory T cells5.
The negative effects of granulocytes on T cells can prolong R&D timelines, complicating the development of optimal CAR constructs or the assessment of therapeutic efficacy. As CAR-T programs transition to GMP manufacturing, contaminating granulocytes can inhibit T cell expansion, which not only jeopardizes the ability to achieve the target number of T cells required for CAR-T production, but raises concerns about granulocyte-induced alterations that can compromise the potency and efficacy of the final CAR-T therapy.
Cryopreservation further exacerbates the issue. Granulocytes are poorly preserved during freezing and are prone to lysis upon thawing. This causes the release of DNA and proteolytic enzymes that affect the viability and functionality of surrounding immune cell populations6.
Mitigating Granulocyte Contamination
Understanding and addressing the factors that influence leukopak quality, such as leukapheresis collection protocols and donor selection can help mitigate granulocyte contamination and quality-related complications to improve CAR-T manufacturing efficiency.
Leukapheresis Collection Protocols
Factors such as collection duration, blood volume, and anticoagulant-to-blood ratio (AC ratio) during collection can influence leukopak quality and the levels of contaminating cell populations2. For cell therapy developers, it’s important to understand these sources of variability and work with vendors who have trained clinical staff and optimized their protocols to ensure the collection of high-quality leukopaks with minimal contamination. Differences across vendor products exist—for instance, BIOIVT reports an average granulocyte content of 5.2% in their non-mobilized leukopaks7, while Charles River‘s non-mobilized leukopaks average 6% granulocytes8. In comparison, Discovery’s AllCells® non-mobilized leukopaks achieve a lower average granulocyte content of 3.3%9.
Allogeneic Donor Selection
Donor selection is equally critical, as individual differences in granulocyte levels can arise because of factors such as genetics, health status, and more. A complete blood count (CBC) test can measure granulocyte content, helping to identify donors more likely to yield high-quality leukopaks with low granulocyte contamination.
Beyond granulocyte content, certain donors possess attributes that make them more ideal for a specific cell therapy. Demographic factors such as age and BMI, as well as biological characteristics such as Human Leukocyte Antigen (HLA) type, KIR (Killer-cell Immunoglobulin-like Receptor) type, or the frequency of a particular immune cell subset, whether it’s a type of T cell, NK cell, or another, and therefore, the collected leukopaks will be more likely to have success in manufacturing2. For example, studies show a positive correlation between initial CD3+ T cell count and CAR-T expansion success. Additionally, donors with less mature and less differentiated T cells at collection are associated with more favorable manufacturing outcomes compared to those with more mature and differentiated T cells.
Vendors with robust donor selection and characterization programs are uniquely positioned to help cell therapy developers identify ideal donors for specific therapeutic programs that can fulfill critical parameters such as achieving sufficient T-cell yield or minimizing contaminating cell types in the leukapheresis product that affect key target cell function. This ensures that only the most suitable and high-quality leukopaks are used in their cellular therapies.
Download the Discovery AllCells® Donor Management Case Studies brochure for more information on how donor selection can set the stage for cell and gene therapy success.
Conclusion
As the CAR-T cell therapy field evolves, the focus remains on accelerating and optimizing manufacturing to make these life-changing therapies more accessible to patients. As outlined here, leukapheresis collection practices and donor selection influence leukopak quality. By adopting standardized protocols and best practices, developers can ensure efficient T cell expansion, shorten production timelines, and improve overall CAR-T product quality.
Given the critical need for consistency and quality in leukopaks, many cell therapy developers choose to partner with commercial vendors over non-profit biobanks. Commercial vendors can provide robust donor characterization programs, stringent collection protocols, and scalable processes as well as also offer continuity from research-use-only (RUO) to GMP leukopaks collected under the same high standards. This minimizes variability and contamination, supporting CAR-T development and manufacturing across all stages, from discovery to commercial production.
While hematological malignancies have been at the forefront of CAR-T therapies, their success has provided a pathway to applications in solid tumors, and non-cancer indications including autoimmune diseases, and neurodegenerative disorders. Additionally, researchers are exploring the potential of applying CARs to other immune cell types, such as NK cells, to further broaden treatment options and address unmet medical needs.
In our next blog of this leukopak applications series, we’ll discuss their role in CAR-NK development.
References
- Goyco Vera D, Waghela H, Nuh M, Pan J, Lulla P. Approved CAR-T therapies have reproducible efficacy and safety in clinical practice. Hum Vaccin Immunother. 2024;20(1):2378543. doi:10.1080/21645515.2024.2378543
- Qayed M, McGuirk JP, Myers GD, et al. Leukapheresis guidance and best practices for optimal chimeric antigen receptor T-cell manufacturing. Cytotherapy. 2022;24(9):869-878. doi:10.1016/j.jcyt.2022.05.003
- 3. Chain J. What’s In The Leukopak Matters For Cell Therapy Manufacturing. Cell & Gene. October 11, 2024. Accessed January 17, 2025. https://www.cellandgene.com/doc/what-s-in-the-leukopak-matters-for-cell-therapy-manufacturing-0001
- 4. Bunse CE, Tischer S, Lahrberg J, et al. Granulocyte colony-stimulating factor impairs CD8(+) T cell functionality by interfering with central activation elements. Clin Exp Immunol. 2016;185(1):107-118. doi:10.1111/cei.12794
- Agashe C., et al. Impact of granulocyte contamination on PBMC integrity of shipped blood samples: Implications for multi-center studies monitoring regulatory T cells. J Immunol Methods. 449; 23-27. Oct 2017.
- Olson WC, Smolkin ME, Farris EM, et al. Shipping blood to a central laboratory in multicenter clinical trials: effect of ambient temperature on specimen temperature, and effects of temperature on mononuclear cell yield, viability and immunologic function. J Transl Med. 2011;9:26. Published 2011 Mar 8. doi:10.1186/1479-5876-9-26
- 7. Rosenberg What is Leukapheresis. BIOIVT. January 27, 2020. Accessed January 17, 2025. https://bioivt.com/blogs/what-is-leukapheresis
- Scott E, Wassim Basheer W and Hansen K. A Comprehensive Analysis of CD34+ Stem Cell Mobilization Regimens. Charles River Laboratories. Accessed January 17, 2025. https://www.criver.com/resources/download-cs-a-comprehensive-analysis-of-cd34-stem-cell-mobilization-regimens



