Counting Protocol
How to Count Cells and Determine Percent Viability

Recommended Counting Method Using an Automated Cell Counter
Materials
- Dual Nucleation Dye, such as Acridine Orange/Propidium Iodide
- Cell Counting Chamber
- Automated Cell Counter
When you receive an AllCells® fresh product, please count the cells immediately upon receipt. Before any further manipulation, cryopreserved products should be counted immediately after thawing. Discovery is not liable for any cell loss during subsequent processing or manipulation.
Counting Cell Products
- Proper aseptic technique and personal protective equipment should always be used when handling cell products.
- Mix the cell suspension thoroughly to ensure even distribution of the cells.
- For fresh cell products shipped in a bag, use both hands to pick up the transfer bag by the top and bottom edges and invert the bag side to side (approximately 1 second for each rotation) for 5 total rotations to mix.
- Wipe the transfer line with an alcohol swab. Make sure the line is clamped before cutting the line with sterilized scissors.
- After the line has been cut, unclamp the line and dispense a 2-3mL aliquot of the cell product into a sterile tube.
- To mix cells, pipette up and down at least 5 times with a P1000 pipetman.
- Dilute the sample as needed for the cell counter being used. For example, for Cellometers, dilute 100ul sample with 900ul DPBS for a 1:10 dilution.
- For fresh cell products shipped in other containers, mix the cells by gently pipetting 25-50% of the cell suspension up and down at least 5 times using an appropriately sized pipettor or serological pipette.
- Dilute the sample as needed for the cell counter being used. For example, for Cellometers, dilute 100ul sample with 900ul DPBS for a 1:10 dilution.
- For cryopreserved cell products, follow the procedure for thawing bags or vials.
- Mix the cell suspension with an equal volume of the appropriate cell counting dye.
- Load the appropriate chamber for the cell counter being used.
- Count the sample per the recommendations of the cell counter, taking into account the initial dilution performed.
Alternative Counting Method Using Trypan Blue:
Materials
- 4% Trypan Blue
- Neubauer Hemacytometer and cover slip
- Dulbecco’s Phosphate buffered saline (DPBS) optional
- After following handling and dilution as described above, immediately take a 10μL aliquot of the cell product from the vial/tube after mixing.
- Combine 10μL of the diluted/undiluted test sample with 10μL 0.4% trypan blue. The test sample should be counted within 5 minutes after mixing with trypan blue.
- Load the hemacytometer with approximately 10μL of the trypan blue-labeled sample. Make sure the entire chamber is filled with the test sample.
- Under bright field, dead cells appear light to dark blue while viable cells appear clear to autofluorescent (Figure 1).
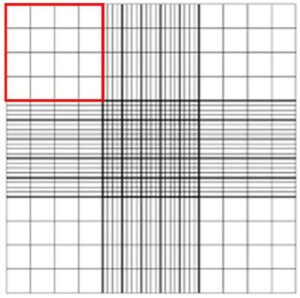
- Count all the viable cells in a quadrant (Figure 2). To achieve better accuracy, ≥ 100 viable cells should be counted. More than one quadrant may need to be counted to achieve ≥ 100 viable cells counted.
- Count the dead cells in the same quadrants.
- Calculations to determine the cell concentration and percent viability.
- Cell Concentration (cells/mL) = (# total viable cells counted / # quadrants counted) x final dilution factor x 10^4*
- Cell Viability (%) = (# viable cells counted / (# viable cells + # dead cells counted)) x 100
Figure 1. Differentiation of live and dead cells using trypan blue dye exclusion. Black arrows indicate live cells and red arrows indicate dead cells stained with dye.
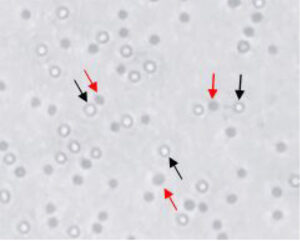
Figure 2. Hemacytometer with Neubauer gradients (from http://www.hausserscientific.com/). A counting quadrant is highlighted in red outline.
How can we help you?
