With the FDA approvals of several cell therapy products (Kymriah® and Yescarta®) and many more cell therapies progressing towards clinical trials, sourcing high-quality cellular materials has become a critical determinant of cell therapy development success. Particularly as allogeneic approaches to cell therapies become more feasible, access to donor cells that meet regulatory, quality, and quantity needs is paramount. Leveraging a supplier’s industry expertise and, more importantly, finding one who can work as part of your team can save money and also expedite timelines to achieve your program goals.
The starting cells from human donors are the most critical component of any cell therapy but they are inherently variable. From preclinical research and development to large-scale commercial manufacturing, identifying suitable raw material suppliers for critical components can save money, time, and, more importantly, ensure success.
What is GMP/GTP?
The main regulatory standard for ensuring pharmaceutical quality is the Good Manufacturing Practice (GMPs). Regulatory agencies, such as the U.S. Food and Drug Administration (FDA), Health Canada, the European Medicines Agency (EMA), and others, are responsible for developing and enforcing GMP standards.
Suppliers of human cellular and tissue-based products are also regulated by Good Tissue Practice (GTP) guidelines for the protocols and facilities used for manufacturing. GTP includes parameters like donor screening and infectious disease testing, product recovery, processing, storage, labeling, and distribution.
Founded by Jay Tong, MD, AllCells is the leading provider of high-quality human primary cells from peripheral blood and bone marrow since 1998. Fresh, GMP clinical grade bone marrow and leukopaks are collected and processed in the California LeukoLab – Donor Care and Management Facilities. These products adhere to rigorous quality management system (QMS) oversight that meets all regulatory standards and protocols.
AllCells’ GMP products meet all current FDA and European Medicines Agency (EMA) guidelines for quality biological products. Donors are screened and tissues are collected in accordance with FDA Title 21 CFR 1271 subparts A-C and provisions of subpart D. AllCells also meets EU directives 2004/23/EC and 2006/17/EC, wherein AllCells is considered a Procurement Organization.
At AllCells, we understand that your cell and gene therapy product is unique. AllCells takes a team approach with stakeholders striving to establish long-term partnerships with researchers, contract research organizations (CROs), or contract development and manufacturing organizations (CDMOs) to deliver Clinical Grade products for allogeneic use. Leverage our team of experts with expertise in drug development, 3rd party, and commercial manufacturing, and all of the regulatory aspects of licensed products to help you meet your program needs.
Donor Management and Screening
Donors are the most important asset to obtaining high-quality cells. AllCells manages the largest donor database in the industry with an extensive registry of healthy, IRB-consenting, recallable donors with diverse demographics and backgrounds to provide researchers with the right donors, with the appropriate clinical parameters, at the right time.
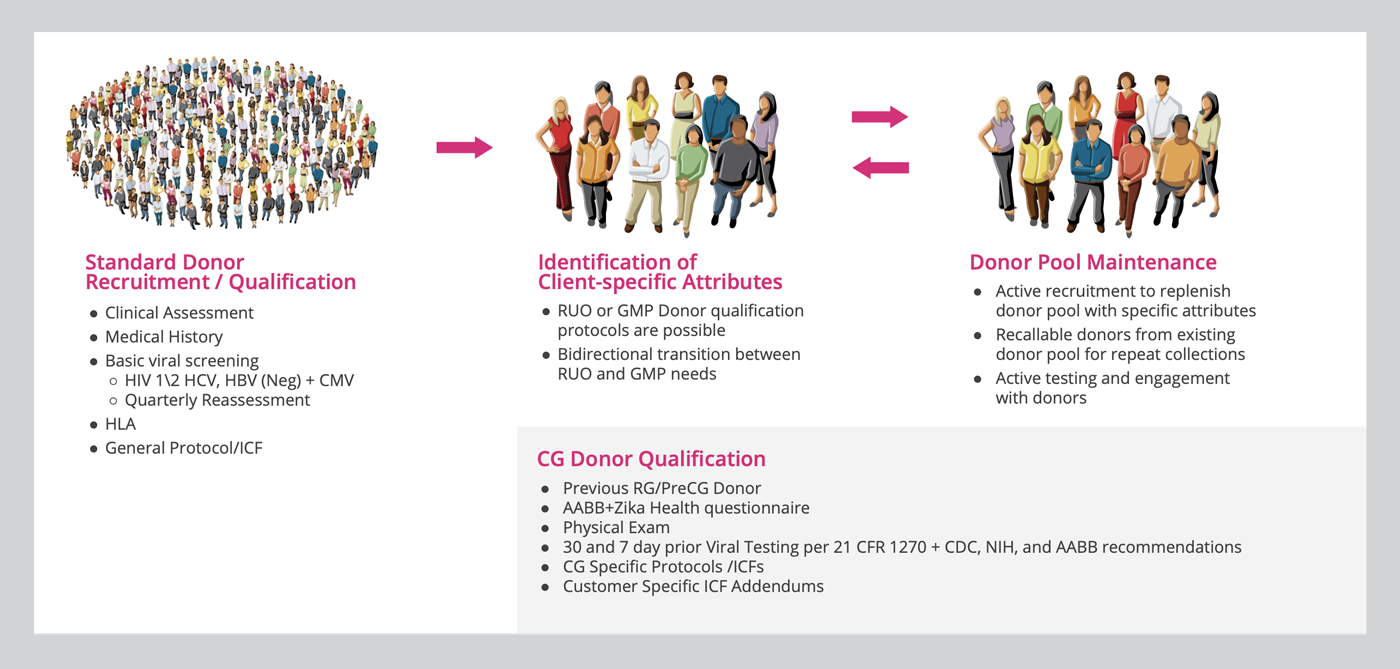
The entire donor qualification process is outlined in Figure 1. After an initial and in-depth screening, all donors undergo a standardized screening process at three-month intervals. Donors are tested against an infectious disease panel prior to donating for research grade cellular products. For clinical grade donations, donors must meet additional, more stringent testing requirements.
- FDA-mandated 21 CFR 1271 viral screening by Clinical Laboratory Improvement Amendments (CLIA)-approved laboratories in compliance with good tissue practices (GTPs) 30 days and again 7 days prior to donation
- American Association of Blood Banks (AABB)-derived health history verifications and physical assessments completed by trained medical personnel
Once the donor is qualified, the GMP cell collections are performed by qualified clinical staff using GMP standard operating procedures and are extensively documented to ensure compliance. Clinical grade products have additional quality assurance oversight, extensive GMP documentation, enhanced aseptic handling and processing, and quality results reporting.
Additionally, a secondary, backup clinical grade donor provides further assurance against the possibility of donor attendance failures and the expenses associated with the unexpected unavailability of cells. AllCells makes sure you can get the cells you need when you need them. We can also help you identify and customize a pool of recallable donors that meet your specifications to ensure supply chain continuity for your workflow.
AllCells GMP Clinical Products
AllCells offers both GMP clinical grade fresh Bone Marrow and Leukopaks:
Fresh Whole Bone Marrow
A rich source of adult stem cells such as CD34+ hematopoietic stem and progenitor cells. Cells from Bone Marrow have shown to have therapeutic potential in translational and regenerative medicine.
Fresh Leukopak
Contains a high concentration of all major lymphocyte populations: T cells, B cells, NK cells, and monocytes.
All of the products are quality controlled for cell count and viability using a validated AO/PI protocol to ensure our GMP products meet strict quality specifications. Sterility testing of our fresh Bone Marrow and Leukopak Clinical Grade products is performed through compendial testing by qualified third-party laboratories as specified by USP 71.
Final Remarks
Autologous therapies will continue to progress at the forefront of the field but allogeneic approaches are gaining momentum. This will lead to an increased demand for a reliable, consistent supply of cellular material from donors, which are the starting raw materials needed to manufacture these therapies. Utilizing GMP cells sourced from well-characterized, viral-tested donors ensure consistency and quality standards can be met. This saves time and money not only in the transition between research to clinical development but also through large-scale commercial manufacture since the entire workflow has utilized regulatory compliant materials.
AllCells’ comprehensive operational facility standard operating procedures, quality management systems, quality assurance audits, documented training, GMP collection documentation, a localized chain of custody from donor to shipment, validated product release assays, compendial testing, rigorous QA review, record management and demonstrated stability ensures the highest quality. Our dedicated team, with years of experience, is here to help you identify and meet your program needs. We are committed to providing high-quality, GMP cell material for further manufacture of cell therapies.