The success of chimeric antigen receptor (CAR)-T cell therapy has spurred interest in applying CAR technology to other immune cells in hopes of addressing some known limitations. With their intrinsic immune activity and favorable safety profile, natural killer (NK) cells have enormous potential for adoptive cellular therapy1.
Unlike T cells, which require antigen sensitization and major histocompatibility complex (MHC) engagement, NK cells can recognize and eliminate malignant cells through multiple mechanisms. These include direct cytotoxicity via cytolytic protein release, antibody-dependent cellular cytotoxicity (ADCC), and the ability to recruit other immune cells to amplify immune responses1-3.
A search of clinicaltrials.gov shows there are over 160 clinical trials evaluating CAR-NK cell therapies at various stages of development. Significant milestones in 2024, including the FDA trial approval for ImmunityBio’s CAR-NK therapy targeting CD19 for non-Hodgkin’s lymphoma (NHL), highlight the accelerating pace of CAR-NK innovation⁴.
Advantages over CAR-T Cell Therapy
Many CAR-NK development programs rely on leukopaks as a key starting material for isolating NK cells. As with CAR-T cell therapy, the quality and consistency of these collections directly impact the functionality, safety, and manufacturability of the final therapeutic product. By equipping NK cells with CAR constructs, researchers can enhance their cytotoxic potential and target specificity, much like CAR-T cells (Figure 1).
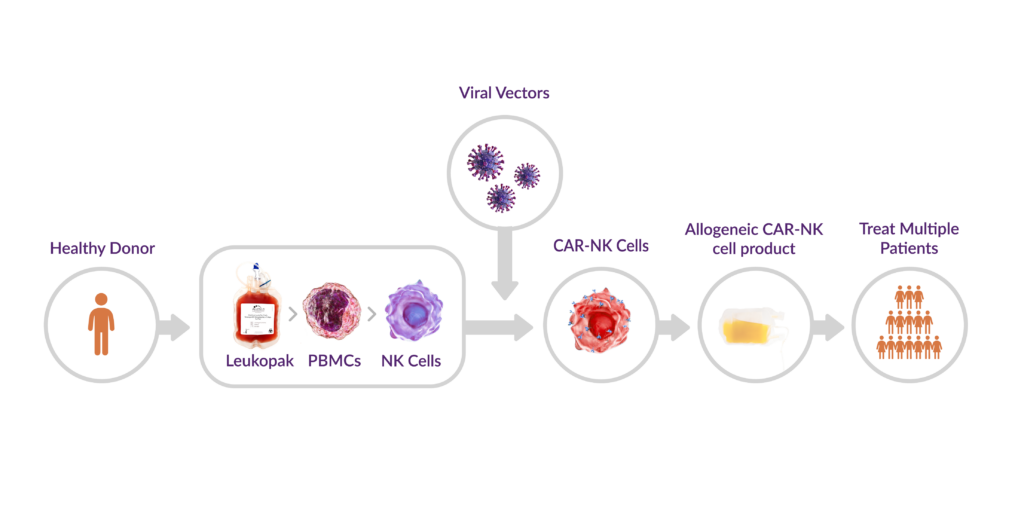
Figure 1. CAR-NK manufacturing workflow
There are several advantages that make CAR-NK approaches appealing alternatives to CAR-T cell therapies, particularly in addressing current challenges such as toxicity, allogeneic applicability, and solid tumor targeting.
Broad Anti-Tumor Activity
NK cells can be engineered with CARs while also retaining their native receptor functions. This dual capability allows CAR-NK cells to eliminate tumor cells that downregulate CAR-specific antigens (i.e., CD19) to evade T cell detection. This broader cytotoxic potential may reduce the risk of tumor escape and treatment resistance.
Lower Toxicity
The shorter lifespan of CAR-NK cells in vivo minimizes the risk of toxicities commonly associated with CAR-T therapy, such as cytokine release syndrome (CRS) and graft-versus-host disease (GvHD). This could improve safety profiles and make CAR-NK cell therapy more accessible to critically ill patients who are ineligible for CAR-T due to toxicity concerns.
Off-the-Shelf Potential
Additionally, MHC-independent NK cell activation overcomes donor-recipient HLA matching requirements that limit some allogeneic CAR-T approaches. Together, these advantages support a scalable, off-the-shelf cell therapy model that could lower costs, shorten manufacturing timelines, and improve patient accessibility.
Over the past five years, a growing number of clinical trials have evaluated CAR-NK therapies across various malignancies, fueling optimism for their efficacy in both hematologic and solid tumors². Unlike CAR-T cells, which struggle against solid tumors due to the immunosuppressive tumor microenvironment, NK cells have innate mechanisms that enable them to infiltrate and persist in solid tumors more effectively3. This makes CAR-NK therapy a promising candidate for improving the efficacy of CAR-based immunotherapies for solid tumor cancers.
Optimizing Leukopaks for CAR-NK Development
In the first blog of our leukopak applications series, we discussed how both leukopak quality and donor selection impact CAR-T development. The same considerations apply to CAR-NK therapies, where high-quality starting material is essential for developmental success. Since NK cells are less abundant in peripheral blood than T cells, variability in leukapheresis collection parameters—such as total volume, anticoagulant use, and processing time—can impact NK cell yield and viability. Optimized collection protocols help maximize NK cell collection to achieve a higher recovery of functional cells for downstream CAR-NK manufacturing.
Donor Characterization and KIR Typing
The complexity of cell and gene therapies (CGTs) means donor selection must go beyond basic demographics like age, sex, and BMI. On a cellular level, factors such as HLA and KIR profiles, immune cell frequency, and functional characteristics can significantly influence therapeutic outcomes.
For CAR-NK development, KIR typing—which identifies the presence or absence of genes encoding killer cell immunoglobulin-like receptors (KIRs) on NK cells—is particularly relevant. KIR-HLA interactions influence NK cell function and therapeutic potential5. Emerging clinical evidence suggests that selecting KIR-HLA mismatched donors may enhance anti-tumor activity and improve persistence in vivo6,7. Identifying donors with optimal immune profiles can improve CAR-NK efficacy and durability.
To that end, more developers want to pre-screen donors to create target donor profiles for allogeneic programs. Partnering with a vendor with the capabilities to thoroughly characterize all aspects provides critical data to inform donor selection strategies.
To support this, Discovery Life Sciences’ AllCells® Pre-Characterized Donor Selection Program streamlines the donor selection process. Clients can access a comprehensive characterization data repository to identify donors that meet their CGT program requirements without the delays of recalling donors for screening. This pre-characterization also allows researchers to correlate starting material attributes with final product quality to improve quality, consistency, and efficacy.
Bridging the Translational Gap
The allogeneic potential of CAR-NK therapy makes the quality and consistency of starting materials even more critical, often determining how quickly a program can progress through the investigational pipeline. However, in an industry lacking standardized raw cellular materials, bridging the translational gap can be a significant challenge.
Not all vendors have the specialized expertise or infrastructure to collect leukopaks to GMP standards or to find donors who meet FDA 21 CFR 1271 Subpart C requirements for GMP collection. A vendor with a robust donor ecosystem and deep leukapheresis expertise ensures seamless RUO-to-GMP continuity. Additionally, vendors with AABB-accredited facilities, highly trained clinical staff, and a strong quality framework help ensure quality and regulatory compliance. Without these capabilities, developers risk costly revalidation studies and potential delays that could hinder program progress.
As CAR-NK and other cell-based therapies continue to advance, high-quality leukopaks remain vital for successful manufacturing and clinical outcomes. Beyond cell therapy, they also serve as foundational starting materials for gene editing applications, where precision and consistency are equally critical. In our next blog, we’ll explore how leukopaks are driving innovation in ex vivo gene editing and enabling the next generation of cell and gene therapies.
References
- Riedell, PA. Unlocking the Potential of CAR Natural Killer Cell Therapy in Hematologic Malignancies While Overcoming Challenges. ASCO Daily News. Published October 2, 2024. Accessed February 7, 2025. https://dailynews.ascopubs.org/do/unlocking-potential-car-natural-killer-cell-therapy-hematologic-malignancies-while
- Zhong Y, Liu J. Emerging roles of CAR-NK cell therapies in tumor immunotherapy: current status and future directions. Cell Death Discov. 2024;10(1):318. Published 2024 Jul 10. doi:10.1038/s41420-024-02077-1
- Wrona E, Borowiec M, Potemski P. CAR-NK Cells in the Treatment of Solid Tumors. Int J Mol Sci. 2021;22(11):5899. Published 2021 May 31. doi:10.3390/ijms22115899
- DelveInsights Business Research, LLC (2024). NK Cell Therapies – Pipeline Insight, 2024. https://www.delveinsight.com/report-store/nk-cell-therapy-pipeline-insight
- Khawar MB, Sun H. CAR-NK Cells: From Natural Basis to Design for Kill. Front Immunol. 2021; 12:707542. Published 2021 Dec 14. doi:10.3389/fimmu.2021.707542
- Liu E, Marin D, Banerjee P, et al. Use of CAR-Transduced Natural Killer Cells in CD19-Positive Lymphoid Tumors. N Engl J Med. 2020;382(6):545-553. doi:10.1056/NEJMoa1910607
- Lemar H, Vohra A, Xie M, et al. 128 KIR haplotype can inform donor selection production of allogeneic memory-like CAR NK cells for clinical application. Journal for ImmunoTherapy of Cancer 2021;9: doi: 10.1136/jitc-2021-SITC2021.128



