
AllCells® GMP Mobilized Leukopak
Discovery’s AllCells® GMP Mobilized leukopaks are apheresis products collected from healthy donors treated with FDA-approved drugs to stimulate mobilization of CD34+ hematopoietic stem and progenitor cells from the bone marrow to the peripheral blood. This procedure results in large-scale yields of CD34+ cells. Peripheral blood is collected from IRB-consented donors using the continuous flow Spectra Optia® Apheresis System directly into ACD-A anticoagulant at AllCells’ FDA-registered, IRB-approved collection facilities.
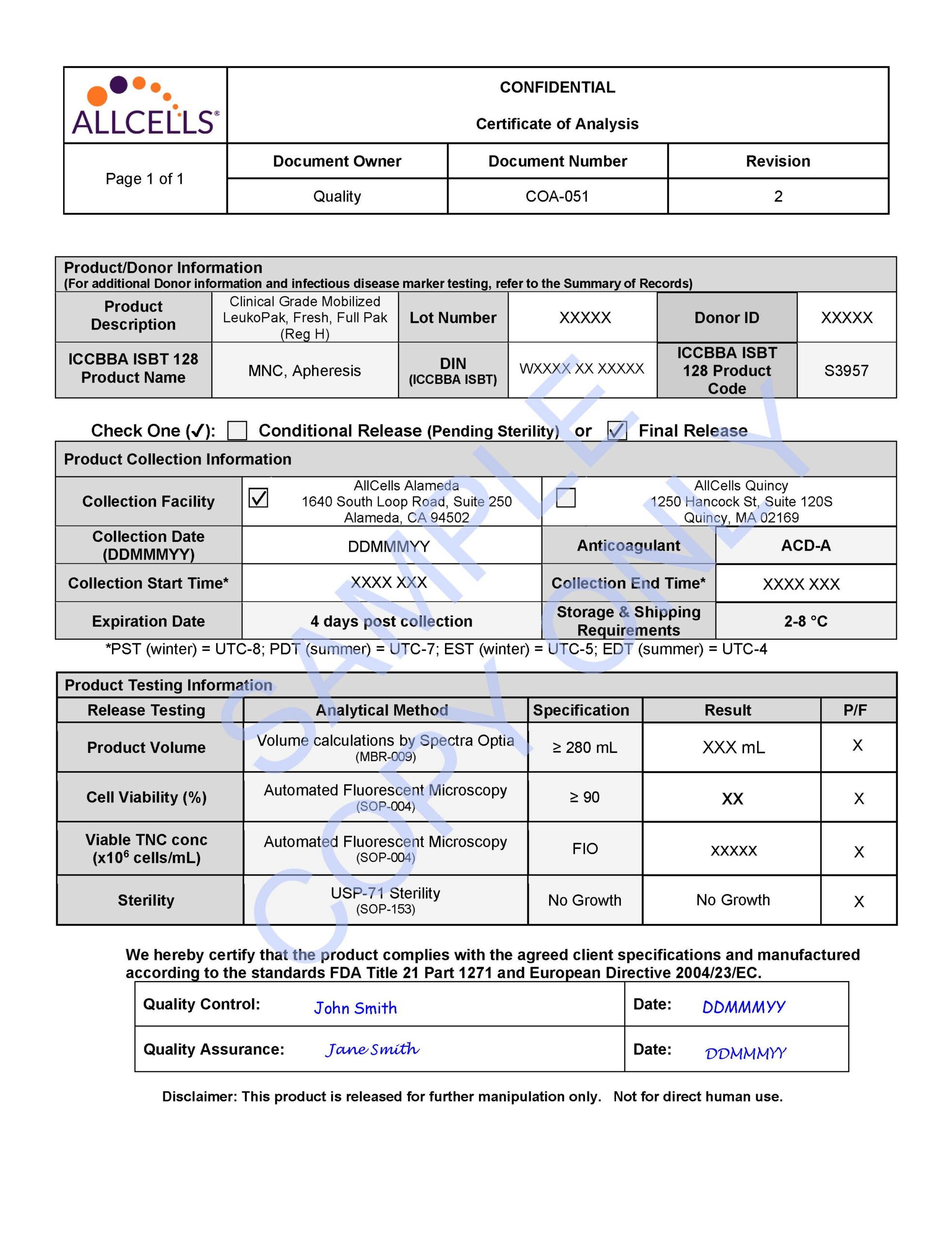
AllCells® GMP Mobilized Leukopaks are enriched in CD34+, allowing for scalability and reproducibility. The product is quality controlled for viability using a validated AO/PI protocol and pass a sterility test in accordance with USP <71> guidelines using compendial testing.
- Catalog Numbers: CG, mLP, RegH, FR, Full Pak and CG, mLP, RegH, FR, Full Pak 2
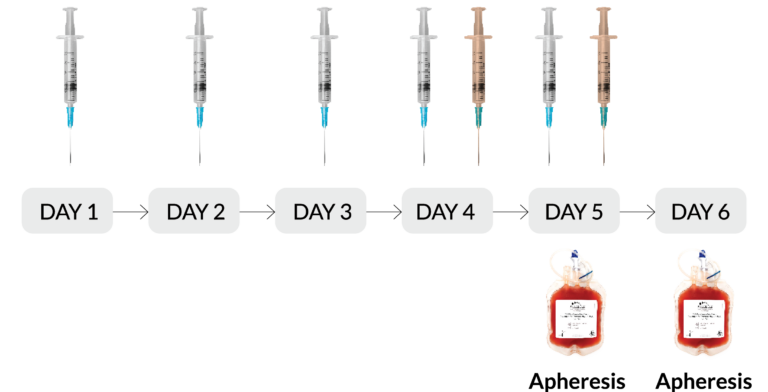
- Mobilization Regimen: G-CSF x 5 days + plerixafor x 2 days (RegH)
- Format: Fresh, 2 Paks
- Anti-coagulant: ACD-A
- Viability: NLT 90%
- Fill Volume: NLT 280mL
- Cell Concentration: FIO
- Sterility USP <71>: No growth
- Shipping Condition: 2° to 8°C in validated shipping container with temperature logger
- Documentation: Certificate of Analysis, Summary of Records
Subcutaneous injection of 10μg/kg/day G-CSF
Subcutaneous injection of 240μg/kg plerixafor
At AllCells, we believe that your success is our success. We believe that sharing our knowledge and expertise is more than a business practice, it’s a responsibility. And we believe that collaboration leads to better outcomes. Below is a collection of relevant links to Leukopak resouces including blog posts, webinars and useful protocols to help you become more efficient and more effective. Have technical questions about Leukopak products? Our Customer Success Team is standing by.
BLOG POSTS
- Navigating the complex world of regulations: How AllCells is changing cell therapy and protecting against product-ending events
- The Key to Developing Good Manufacturing Practice (GMP) Biomaterials for Cell and Gene Therapy Development
- Dual Mobilization Enhances CD34+ Yield Across All Donor Types
- The Therapeutic Potential of HSC-based Gene Therapies
- Why You Should Start Clinical Grade Cell Supply Discussions Early in Cell and Gene Therapy Development
- What You Need to Know About Regenerative Medicine in 2022: Industry and Tech Trends
PROTOCOLS
No related posts found.

AllCells® GMP Mobilized Leukopak Difference
- Seamlessly transition from process development to manufacturing with RUO Mobilized Leukopaks and GMP Mobilized Leukopaks
- Process without delays with 99% deliverability rate of GMP products
- Ensure consistency and robust manufacturing processes with up to 5 mobilized collections from the same donor
Donor Management Services
Donors with the right attributes are a critical part of any cell therapy program. AllCells Donor Management Services can help you find, screen and retain donors from our repository comprised of highly-characterized, HLA-typed donors with diverse demographics and clinical parameters that can meet program-specific criteria from research and development to commercialization.

