Engineered T-Cells: A look at Autologous and Allogeneic CAR-T Cell Immunotherapies

Re-tasking our body’s own immune system to treat cancer has been a major scientific goal over the last few decades. According to the National Cancer Institute, it is estimated that 1,735,350 new cases of cancer were diagnosed, and 609,640 deaths were reported in the US in 20181. One of the most revolutionary approaches to date is chimeric antigen receptor (CAR) T cell therapy, a type of personalized therapy where the body’s own immunological defenders, the T cells, are genetically modified to express CARs, which are specifically targeted to attack cancerous cells. These CAR-T cells are then re-infused back into the patient in a process called adoptive cell therapy for clinical effect. Since their initial development in the 1990s, CAR T cells have evolved through several generations, with researchers fine-tuning the safety and efficacy profiles using novel concepts and technologies.
The landmark approvals of Kymriah® and Yescarta® in 2017 in the United States, both autologous (patient-specific) CD-19 targeted CAR-T cell therapies for the treatment of B-cell malignancies have spurred rapid advancements in the field. Despite the groundbreaking clinical results of autologous CAR-T cell therapy, not all patients are eligible for the procedure and the treatment has some significant pain points such as long, expensive and complex manufacturing processes due to its patient-specific nature. As a result, there has been a shift towards allogeneic (‘non-self’), universal ‘off-the-shelf’ CAR T cell therapies. CAR-T cell immunotherapy has emerged as a leading curative modality for relapsed hematological malignancies and has ushered in an exciting new era of cancer treatment.
Background
As its name implies, the backbone of CAR-T cell therapy are T cells, which are lymphocytes or white blood cells involved in cell-mediated immunity. T cells are distinguishable from other lymphocytes by the presence of a T-cell receptor (TCR). Fragments of foreign antigens from an infected or foreign cell are presented to T-cells in the context of specific major histocompatibility complex I (MHC class I) molecules, called human leukocyte antigens (or HLAs). Once the antigen is bound to the TCR, an intracellular signaling cascade results in T cell-mediated apoptosis of the target cell. This activation of T cells occurs in an MHC-dependent manner, which is necessary for the immune system to differentiate ‘self’ from ‘non-self’.
Researchers have long been interested in harnessing the power of T cell-mediated cytotoxicity in a directed fashion and there has been success in using engineered TCRs targeted against specific tumor antigens with promising therapeutic effect against certain malignancies; however, the mechanism of T cell activation is a limiting factor for their broad use in adoptive cell therapies. One way that cancer cells escape detection and destruction by T cells is through downregulating their HLA expression. The development of chimeric antigen receptors (CARs) provides a more universal approach to cancer immunotherapy because unlike a normal TCR, CARs are engineered to function independent of the HLA complex for antigen recognition and binding since the antigen specificity is defined by a high affinity antibody domain. This allows the CARs to not only recognize peptide antigens but any target for which an antibody is available (i.e. glycolipids or carbohydrates)2,3. Additionally, by circumventing the MHC dependence and MHC class I and II restriction, both CD8+ and CD4+ T cell subsets can be engineered with CARs for target cell recognition.
CARs are composed of three parts and have undergone step-wise development to improve their persistence and cytotoxicity (Figure 1) :
- Extracellular antigen-binding domain derived from a tumor-specific monoclonal antibody single chain variable fragment (scFv)
- A transmembrane domain anchors the CAR to the T cell; derived from CD3, CD4, CD8 or CD28
- An intracellular T cell activation domain of CD3ζ with one or more costimulatory domains (usually 4–1BB or CD28)
Upon scFv binding to a pre-specified antigen, the intracellular CD3ζ domain activates intracellular events in the T cell to induce tumor-specific cytotoxicity.
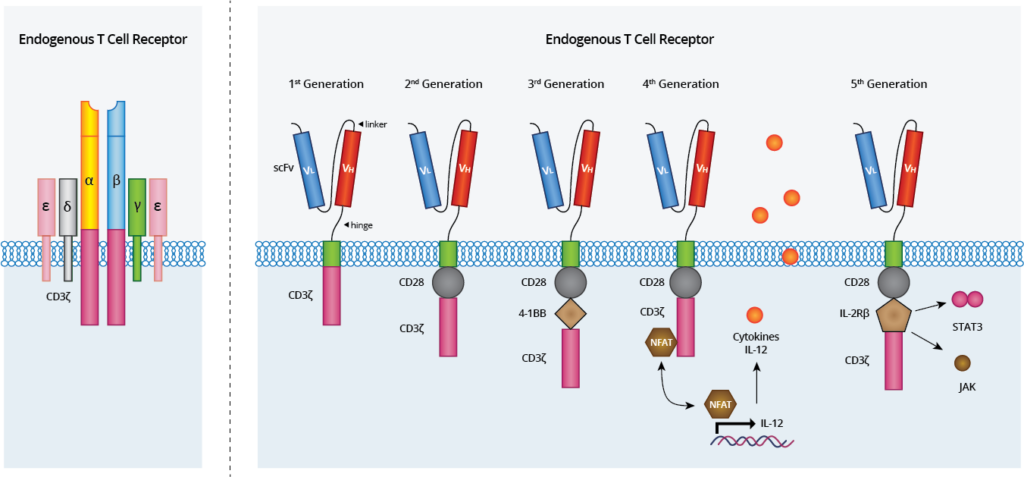
Figure 1. Anatomy of endogenous T cell receptors (left) and Chimeric Antigen Receptors (right). The TCR complex with TCR-α and TCR-β chains, CD3 and ζ-chain accessory molecules. The CARs have evolved from the 1st generation, which contained only CD3ζ as the stimulatory molecule for T cell activation. 2nd generation CARs included one costimulatory domain (i.e. CD28) whereas the 3rd generation CARs consist of two domains linked to CD3ζ (i.e. CD28 and 4-1BB), improving CAR-T cytotoxicity and durability. The 4th generation of CARs are based on 2nd generation CARs paired with gene cassettes for cytokines (i.e. IL-12) production under the control of the NFAT transcription factor. The 5th generation CARs are also based on the second generation of CARs, with the addition of a JAK-STAT activation domain derived from IL-2Rβ. This domain stimulates cell proliferation, prevents terminal differentiation, and shows better persistence.
Currently, there are two main sources of T cells that can be engineered into CAR T cells: those derived from a patient (autologous) and those derived from a healthy donor (allogeneic). In both cases, viral vector transduction, either Ɣ-retroviral vector or lentiviral vector, are most commonly used to insert DNA constructs into the T cell genome which transforms the normal T cells into CAR T cells. More recently, genome editing tools such as CRISPR/Cas9 have also been used to achieve this genetic integration4.
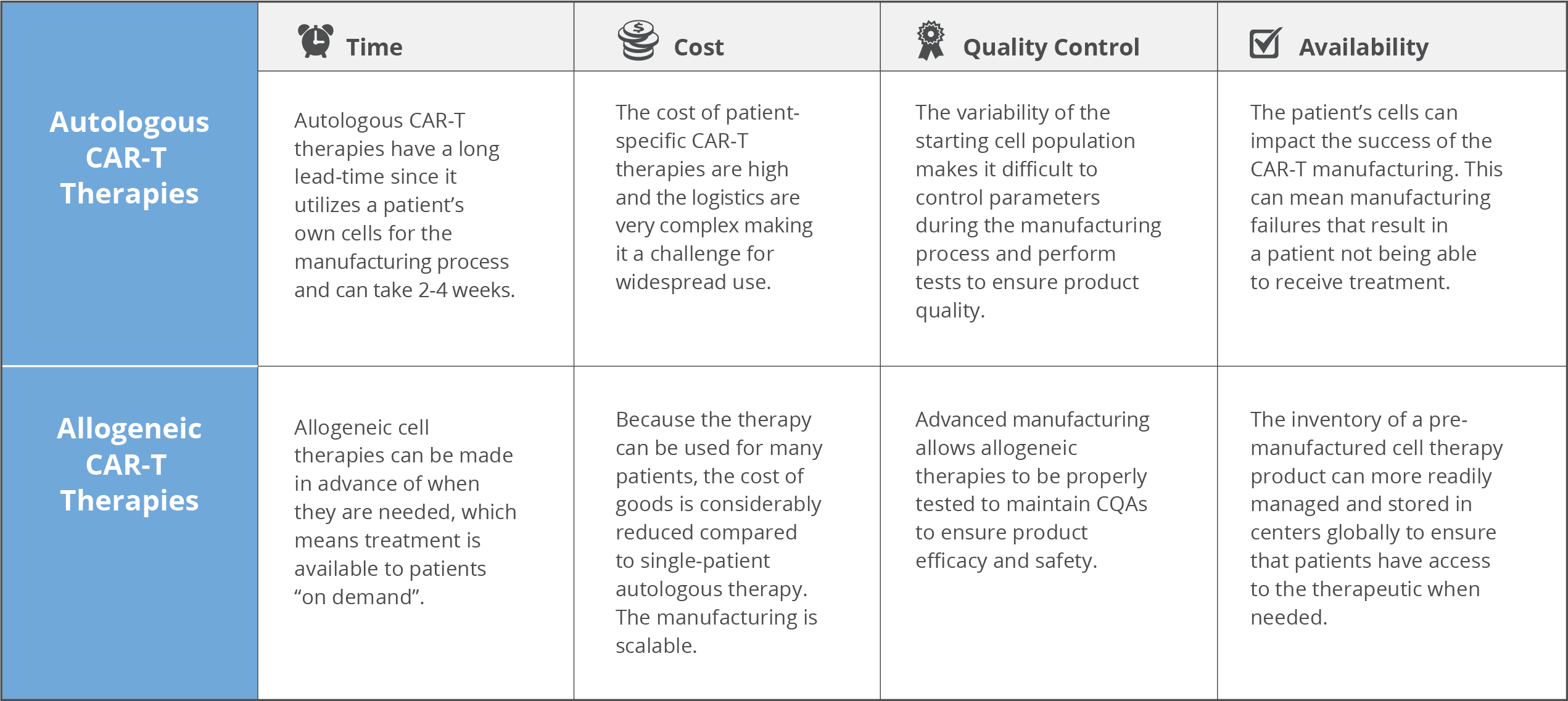
Autologous CAR-T cell therapies have generated impressive clinical results with complete remission rates in B-acute lymphoblastic leukemia in over >80% of patients5. However, some key pain points with autologous cells like cost, complex logistics, reliability and the potential of serious side effects warrants consideration and are current roadblocks to more expansive use of the therapy.
Autologous Therapies
In 2017, the U.S. FDA approved the first two CAR-T cell therapies: Novartis’ Kymriah® for treatment of acute lymphoblastic leukemia (ALL) and Gilead subsidiary Kite Pharma’s Yescarta® for certain types of large B-cell lymphomas, a type of non-Hodgkin lymphoma. These autologous therapies are patient-specific where the therapeutic CAR-T cells are created from a patient’s own cells.
Briefly, a patient’s T cells are collected via leukapheresis and subsequently transported to a manufacturing facility for ex vivo genetic modification. First, the leukapheresis material undergoes enrichment of target T cells using positive selection of CD4 and CD8 markers. Then the T cells undergo activation and viral transduction with CARs targeted against a specific tumor antigen (i.e. CD-19). The transduced cells are culture-expanded, formulated and cryopreserved. This final formulated CAR-T product is then shipped back to the treatment center, thawed and administered to the patient via infusion as a single dose. This complex manufacturing process is compounded by a time-sensitive logistical matrix with many stakeholders and points of potential failure. These and other factors are challenges to be overcome with autologous CAR-T therapies.
Cost
This patient-specific autologous T cell production platform is very costly with Kymriah® costing $475,000 and Yescarta® at $373,000 per patient. The infrastructure, materials, time and skilled labor required to generate these individualized therapies all contribute to the staggering price tag. And because one batch is only one treatment per patient sample there is no economy of scale that can be leveraged.
Time/Complex Logistics
Oftentimes, CAR-T therapy is the last treatment option for critically ill patients. For autologous treatment, the generation of a therapeutic product requires a lengthy and individualized manufacturing process. The entire process of patient apheresis to manufacturing facility and back to the treatment center necessitates a “high touch”, complex network of transportation, scheduling and skilled labor to be successfully executed. This cascade of events is highly subject to delays that have huge ramifications that impact patient outcomes. This can result in extended “vein to vein” times of two to four weeks (or more), which may not be tolerable due to the disease progression of the patient.
Quality of Cells
Typically, patients are critically ill and are severely lympho-depleted from the disease process or previous treatments (chemotherapy, radiation therapy, and/or stem cell transplantation), which compromises the quality and quantity of T cells that can be collected upstream during apheresis. Typically, a therapeutic dose of 107 – 109 cells CAR-T cells is required for treatment6. Therefore, if a patient has a low T cell count or if their cells fail to activate during manufacturing, it can be difficult to produce a sufficient number of CAR-T cells. Novartis’ Kymriah® has shown manufacturing failure rates as high as 9%7. This is especially devastating for patients waiting for CAR-T treatment that they may never receive if the manufacturing process fails. As well, the inability to create inventory with these individualized therapies means that re-dosing may not be possible.
Because cells are manufactured for each individual patient from their apheresis collection, every manufactured batch is unique making it impossible to have a standardized end product. Critical quality attributes (CQAs) that are normally important to control in traditional manufacturing workflows such as cell number, viability, purity, and CAR expression will be highly variable from batch to batch. The resulting product heterogeneity and lack of reference standards complicate the evaluation of safety and efficacy8.
Side Effects
While not fatal in most cases, cytokine release syndrome (CRS) is the most common side effect of CAR-T resulting from the activation of a large number of T cells releasing large quantities of inflammatory cytokines, primarily IFN-γ, IL-6, IL-10, TNF-α and IL-2, several hundred times above baseline. This flood of cytokines causes systemic inflammatory responses, hypotension, fever, and neurological changes. Ironically, CRS is an “on-target”/ “off-tumor” effect of this therapy because the cytokine release indicates the treatment is working; there are active CAR-T cells in the body. The administration of the drug tocilizumab (Actemra®), which blocks IL-6 activity, used to treat juvenile arthritis has been found to treat CRS in patients. Researchers are continuously working to improve the safety profiles of CAR-T therapies to mitigate expected and unexpected toxicities. For example, integration of a “suicide gene” in the CAR may allow better control over the infused T cell population and allow for selective depletion of CAR-T cells if toxicities arise and treatment needs to be halted9. These and other strategies may be essential in the evolution of this technology to ensure patient safety.
Due to these limitations, there is a drive to investigate allogeneic CAR-T therapies as an alternative where T cells would be obtained from healthy donors rather than the patients who are ill. This would minimize variability, streamline processes and timelines to provide a cheaper, more readily available “off the shelf” cell product.
Allogeneic CAR-T Therapy
There are a number of advantages to using allogeneic CAR-T cells including reduced cost of goods, a more simplified supply chain and better characterization/ quality testing of the start and end products, which are common issues associated with autologous CAR-T cells.
Cost
In contrast to an autologous product where each batch is unique and only useful for a specific patient, pre-screened allogeneic donor cells can be used to make CAR-T cells to treat many patients. It is also possible to generate many therapeutic doses from a single manufacturing lot that could be readily available to treat multiple patients (or provide retreatment if required). This drives down the cost of treatment per patient substantially compared to autologous therapies. A study published in 2018 suggests that allogeneic CAR-T could be made at-scale for $7,500-$10,000 per dose6.
Time
Because the allogeneic CAR-T product can be manufactured ahead of time, it can be manufactured at large scale, be properly quality control tested for safety and potency and cryopreserved until such time that it is needed by a patient. This provides the advantage to CAR-T manufacturers the ability to seek out suppliers that can provide high quality donor material since this parameter directly impacts product quality. The “off the shelf” allogeneic CAR-T format minimizes “vein to vein” time, decreases the risk of manufacturing failures and mitigates the complex logistics surrounding patient eligibility, apheresis, manufacturing and treatment that is typical in the autologous framework.
Logistics
With such interest in CAR-T therapies and many researchers and commercial entities working on novel strategies, the demand for high-quality T cells is on the rise. This precursor material is typically the most variable and the most important determinant of success for CAR-T whether you are in early stage clinical development, or in clinical/commercial manufacture. Reliance on a small, local donor pool, while adequate for early phase investigations and development, is not workable for large-scale process development, for which a steady supply of starting material from healthy donors is needed10. It is therefore essential to identify a reputable and suitable raw material supplier to secure this critical component of your supply chain.
AllCells is a leading supplier of research grade and Good Manufacturing Practice (GMP) clinical grade products are used globally by researchers and bio-manufacturers for all stages of product development, from upstream, pre-clinical testing through to large-scale commercial manufacture of your CAR-T product. AllCells utilizes a unique donor recruitment and screening system to manage a large repository of high quality donors through their on-site, Institutional Review Board (IRB)-approved state-of-the-art bi-coastal donor collection facilities. These donors for the GMP clinical grade products are screened and tissues are collected in accordance with CFR 21 Part 1271 and EU directives 2004/23/EC and 2006/17/EC, designed specifically for further manufacturing of allogeneic cell-based therapies. Additionally, the established relationships that AllCells has with its donor pool can be leveraged to request repeat collection from key ‘high-value’ HLA-specific donors, who may have desirable attributes such as the ability to provide large volume leukapheresis collection with high therapeutic cell counts11.
The AllCells team strives to establish long-term partnerships with researchers, contract research organizations (CROs) or contract development and manufacturing organizations (CDMOs) to source the right material on demand and maintain that supply of quality cells. The team also has expertise in drug development, third party and commercial manufacturing, and a deep understanding of the regulatory aspects of licensed products to help you meet your program needs. Continuous process improvements to fine-tune cell/tissue isolation protocols, optimize storage temperature conditions and evaluating cryopreservation media (where applicable; for research-grade products) to improve the stability and extend shelf-life of the cells is always ongoing to bring the highest quality, most reliable products to market.
Another important logistical consideration is the transportation of cells to centralized manufacturing facilities. After the source material is isolated, cells need to be processed, packaged and shipped to the manufacturing site where further processing and isolation, selection and expansion, and purification steps occur. It is beneficial to partner with an experienced company like AllCells who work with cold-chain experts like Aerosafe Global™. Aerosafe’s quality-assured and temperature-controlled packing system is used to ship AllCells’ GMP Clinical Grade products to a single or multi-site manufacturing location(s) globally. This system can maintain a safe temperature range for up to 96 hours post-packaging to accommodate any unexpected shipping delays without compromising on product stability and efficacy.
Quality of Cells
One of the biggest challenges with autologous CAR-T therapies is the starting cell population from the patient is highly variable in quality due to a number of factors. The benefit of allogeneic CAR-T therapies is that the highest quality donor cells can be sourced, which directly relates to the quality of the resulting therapeutic. When using a healthy donor, he/she can undergo specific prescreening for desirable attributes like number of T cells, phenotype and CD4:CD8 ratio optimal for CAR-T to minimize manufacturing failures11. Selection of donors who have a high percentage of T cells is desirable since the contaminating non-T cell populations can impact downstream manufacturing. The more control over the input cells, the greater the robustness of manufacturing processes to create a consistent and homogeneous product.
At present, no uniform quality testing standards exist for the final CAR-T cell products due to differences in the CAR design, gene introduction, cell culture, and cell purification technologies from each manufacturer11. That said, it is important that manufacturers follow GMP guidelines to maintain traceability between donors and collected raw materials, equipment, and uphold labor force training standards to ensure a high quality product12. Critical Quality Attributes (CQAs) should generally include product identification, biological efficacy, purity, impurities, cell number and general detection (i.e. sterility, mycoplasma, endotoxin, and appearance)13,14.
Challenges
One of the biggest complications associated with allogeneic CAR-T cell therapy is the development of graft-versus-host-disease (GvHD)8,9. This occurs when the T cell αβ receptor (TCRαβ) on the infused allogeneic CAR‐T cells recognize cell surface HLA class I and class II molecules on the recipient’s cells as “non-self”, which triggers an undesirable systemic immune response. Therefore, circumventing GvHD is paramount to the broad utility of allogeneic CAR-T therapy particularly when the CAR-T cells infused into a patient are from HLA-mismatched donors.
Researchers and pharmaceutical companies invested in allogeneic CAR-T therapies are using gene-editing tools like zinc finger nucleases (ZFNs), transcription activator like effector nucleases (TALENs) and CRISPR/Cas9 to knock out TCRαβ expression in the donor cells to remove the HLA barrier altogether to produce a truly universal CAR-T product8,9.
Final Remarks
It is evident that there is a strong academic and industry-driven desire to produce safe, ready‐made, “off‐the‐shelf”, efficacious CAR-T cell therapies. Servier’s universal CAR T cells (UCART19) have, in early clinical trials, proven to be a safe treatment for relapsed and refractory CD-19+ B cell ALL with exceptionally low GvHD rates13. The UCART19 cells are gene-edited to knock out expression of the TCR through disruption of the TRAC loci as well as the CD52 gene using TALEN technology. This dual strategy mitigates the development of HLA-mediated GvHD and improves the persistence of donor UCART19 cells after lymphodepletion with the drug Alemtuzumab (anti-CD52)13. The TCR-CD52- donor CAR-T cells are unaffected and can seek out target tumor cells without eliciting GvHD, a main concern of allogeneic therapies. These studies, along with other ongoing allogeneic CAR-T trials will prove out this immunotherapeutic for large-scale clinical application.
One key takeaway is that your CAR-T product is only as good as the quality of your starting cells. The potential knock on effects can greatly impact the success of downstream manufacturing. It is imperative to secure a reputable supplier for your cell products, like AllCells, whose cooperation and participation will help to realize your research or manufacturing needs as the field continues to diversify and expand to other targets and indications, including solid tumors. This, in conjunction with improvements to optimize, streamline and decrease the cost of the CAR-T manufacturing will ease concerns and enable continued advances of this revolutionary technology for cancer treatment.
Footnotes
- Cancer Statistics. https://www.cancer.gov/ National Cancer Institute. 27 April 2018. https://www.cancer.gov/about-cancer/understanding/statistics
- Chmielewski, Markus et al. “Antigen-Specific T-Cell Activation Independently of the MHC: Chimeric Antigen Receptor-Redirected T Cells.” Frontiers in immunology vol. 4 371. 11 Nov. 2013, doi:10.3389/fimmu.2013.00371
- Stoiber, Stefan et al. “Limitations in the Design of Chimeric Antigen Receptors for Cancer Therapy.” Cells vol. 8,5 472. 17 May. 2019, doi:10.3390/cells8050472
- Liu, Jie et al. “Building Potent Chimeric Antigen Receptor T Cells With CRISPR Genome Editing.” Frontiers in immunology vol. 10 456. 19 Mar. 2019, doi:10.3389/fimmu.2019.00456
- Graham, Charlotte et al. “Allogeneic CAR-T Cells: More than Ease of Access?” Cells vol. 7,10 155. 1 Oct. 2018, doi:10.3390/cells7100155
- Jenkins, Michael and Farid, Suzanne. Cost-effective bioprocess design for the manufacture of allogeneic CAR-T cell therapies using a decisional tool with multi-attribute decision-making analysis. Biochem Eng Journal vol 137 192. 15 Sept. 2018, doi: 10.1016/j.bej.2018.05.014
- Seimetz, Diane et al. “Approval of First CAR-Ts: Have we Solved all Hurdles for ATMPs?.” Cell Medicine vol. 11 2155179018822781. 22 Jan. 2019, doi:10.1177/2155179018822781
- MacLeod, Daniel T et al. “Integration of a CD19 CAR into the TCR Alpha Chain Locus Streamlines Production of Allogeneic Gene-Edited CAR T Cells.” Molecular therapy: the journal of the American Society of Gene Therapy vol. 25,4 949. 5 Apr. 2017, doi:10.1016/j.ymthe.2017.02.005
- Bonifant, Challice L et al. “Toxicity and management in CAR T-cell therapy.” Molecular therapy oncolytics vol. 3 16011. 20 Apr. 2016, doi:10.1038/mto.2016.11
- Juliano, Lou et al. “The Importance of Collection, Processing & Biopreservation Best Practices in Determining CAR-T Starting Material Quality.” Cell Gene Therapy Insights vol 4, 4 327. 29 May 2018, doi: 10.18609/cgti.2018.032
- Li, Yonghong et al. Quality control and nonclinical research on CAR‐T cell products: general principles and key issues. Engineering. Vol 5,1 122. Feb. 2019, doi: 10.1016/j.eng.2018.12.003
Gee, AP. Manufacturing genetically modified T cells for clinical trials. Cancer Gene Ther, vol. 22,2 67. 22 Mar. 2015, doi: 10.1038/cgt.2014.71 - Zhao, Juanjuan et al. “Clinical trials of dual-target CAR T cells, donor-derived CAR T cells, and universal CAR T cells for acute lymphoid leukemia.” Journal of hematology & oncology vol. 12,1 17. 14 Feb. 2019, doi:10.1186/s13045-019-0705-x
- Wang, Xiuyan, and Isabelle Rivière. “Clinical manufacturing of CAR T cells: foundation of a promising therapy.” Molecular therapy oncolytics vol. 3 16015. 15 Jun. 2016, doi:10.1038/mto.2016.15