
AllCells® GMP Fresh Leukopak
Discovery’s AllCells® GMP Fresh Leukopaks are collected by apheresis from peripheral blood of healthy donors. Our Leukopaks contain a high concentration of lymphocyte populations, T Cells, B Cells, NK Cells, and monocytes. AllCells maintains on-site, FDA-registered donor centers for the collection of all products from IRB-consented healthy donors providing customers with a streamlined process for procuring starting cellular materials for clinical and commercial manufacturing. A Certificate of Analysis (CoA) and summary of record (SoR) are provided with every order.
AllCells® GMP Leukapheresis Products are enriched in single-donor mononuclear cells (MNCs), allowing for scalability and reproducibility of manufacturing process. Our products are quality controlled for cell concentration and viability using a validated AO/PI protocol and pass a sterility test in accordance with USP <71> guidelines using compendial testing.
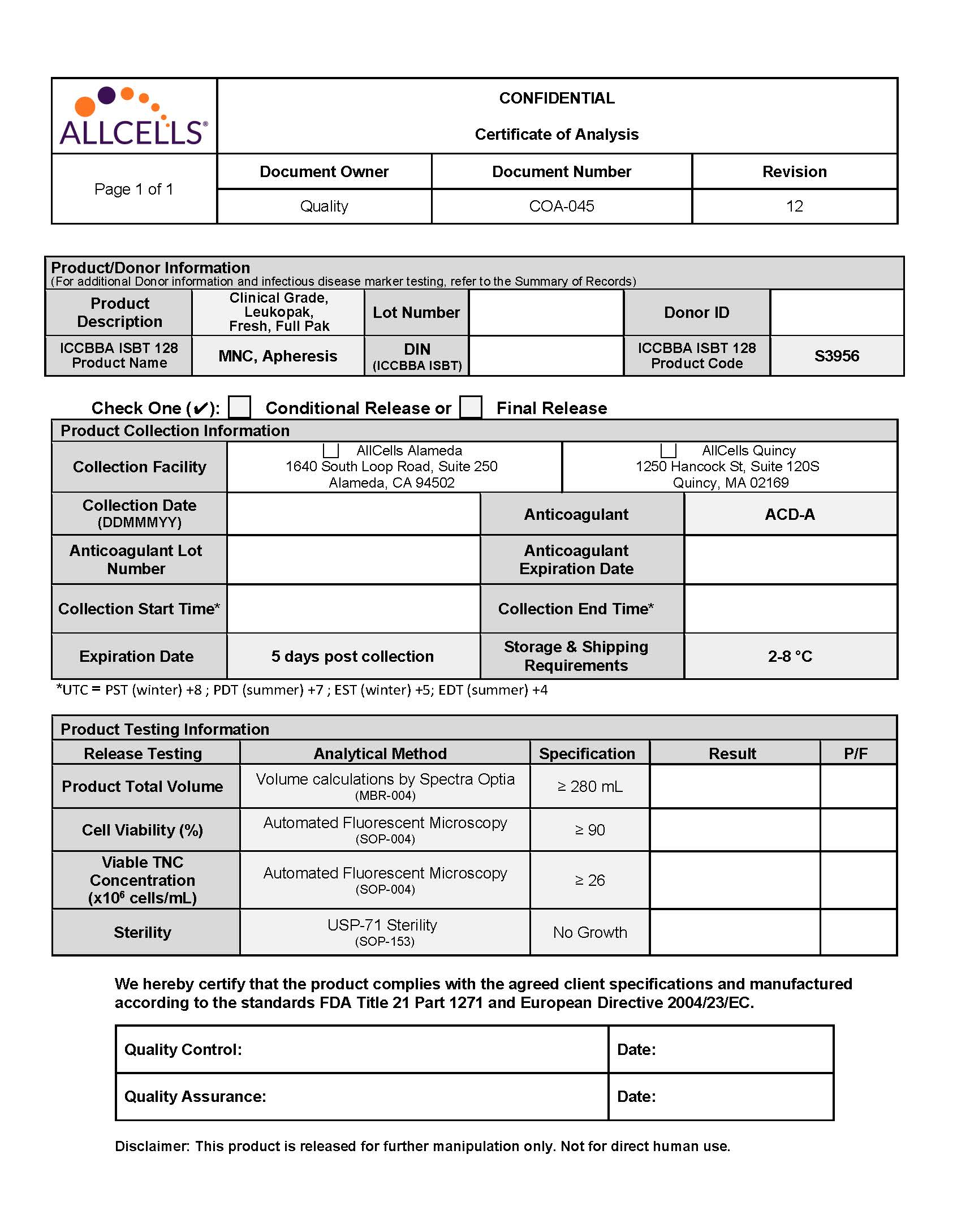
- Catalog Number: CG, LP, FR, Full Pak
- Anti-Coagulant: ACD-A
- Volume: NLT 280mL
- Viability: NLT 90%
- TNC Concentration: NLT 26×106 cells/mL
- Sterility Test USP <71>: No Growth
- Shipping Condition: 2° to 8°C in validated AeroSafe shipping container with temperature logger
- Documentation: Certificate of Analysis, Summary of Records
At AllCells, we believe that your success is our success. We believe that sharing our knowledge and expertise is more than a business practice, it’s a responsibility. And we believe that collaboration leads to better outcomes. Below is a collection of relevant links to Leukopak resouces including blog posts, webinars and useful protocols to help you become more efficient and more effective. Have technical questions about Leukopak products? Our Project Managers are standing by.
WEBINARS
- Catalyzing Market Approval & Driving Sustainable Manufacturing of Allogeneic CGT through Healthy Donor Materials
- Two Essential Aspects to Ensuring the Success of Allogeneic Therapies
- The Importance of Good Manufacturing Practices (GMP) Raw Material Suppliers
- Primary Cells 101: A webinar on human and animal-derived primary cells
- Using Flow Cytometry to Analyze Rare Hematopoietic Cell Subpopulations Implicated in Disease
- In vitro human primary cell based screening assays for vaccine adjuvant development
BLOG POSTS
- Navigating the complex world of regulations: How AllCells is changing cell therapy and protecting against product-ending events
- The Key to Developing Good Manufacturing Practice (GMP) Biomaterials for Cell and Gene Therapy Development
- Overcoming the Global Biomaterial Supply Chain Challenge for the Development of CAR-T Therapies
- Why You Should Start Clinical Grade Cell Supply Discussions Early in Cell and Gene Therapy Development
- A Donor-Centric Approach to Cell and Gene Therapy
- FAQ Series: The Science Behind Cryopreservation
PROTOCOLS

AllCells® GMP Fresh Leukopak Difference
- Seamlessly transition from process development to manufacturing with RUO Fresh Leukopaks and GMP Fresh Leukopaks
- Process without delays with 99% deliverability rate of GMP products
- Maximize downstream manufacturing success by effectively screening, selecting, and retaining donors with desired attributes
Pre-Characterized Donor Selection Program
Discovery’s AllCells® Pre-Characterized Donor Selection Program is designed to expedite the donor selection process to accelerate the advancement of cell and gene therapies to the clinic and beyond. We obtain cryopreserved mononuclear cells (MNCs) from GMP eligible donors whose donor attributes, HLA (human leukocyte antigen) and KIR (killer cell immunoglobulin-like receptor) type have been annotated. Immunophenotyping is performed on the cryopreserved MNCs using our integrated analytical capabilities.

