


AllCells® Leukopaks
Discovery’s leukopaks are collected from healthy and consenting donors using the Spectra Optia® Apheresis System following an IRB-approved protocol. Leukopaks typically contain up to 50% T cells, 20% monocytes, 10% B cells, 10% NK cells, 3% granulocytes, and 3% hematocrit.
We offer leukopaks and isolated cells from leukapheresis material, resulting in higher yields of single-donor mononuclear cells (MNCs) compared to venipuncture collection or buffy coat isolation. This process reduces donor-to-donor variability, allowing for greater scalability and reproducibility of experiments across various applications.
- Whole Apheresis Tissue
- Isolated Cells: Peripheral Blood Mononuclear Cells (PBMCs), T Cells, B Cells, NK Cells, and Monocytes
Leukopak Formats, Attributes, and Unit Sizes
Discovery offers fresh and cryopreserved Leukopaks in a variety of types and sizes. The product chart below provides detailed information on standard formats and unit sizes.
| Fresh | Cryopreserved |
|---|---|
By cell count in Total Nucleated Cells (TNCs): 10 Billion, 5 Billion. 2.5 Billion. By volume: Full Pak, Half Pak, Quarter Pak | 2.5-3.0 Billion TNCs per bag, multiple bags available per donor. Full Pak, Custom |
Don’t see what you’re looking for? Our Leukopak products can also be made-to-order (MTO) with custom specifications to meet the specific requirements of your research project. For more information on Leukopak MTO, please visit our MTO page or reach out to our Customer Success Team. Need immediate help? Chat with a live agent now.
Cryopreserved Leukopaks

Cryopreserved leukopaks and isolated cells offer you the flexibility to perform experiments according to your timeline.
GUARANTEED-CELL-COUNT LEUKOPAKS
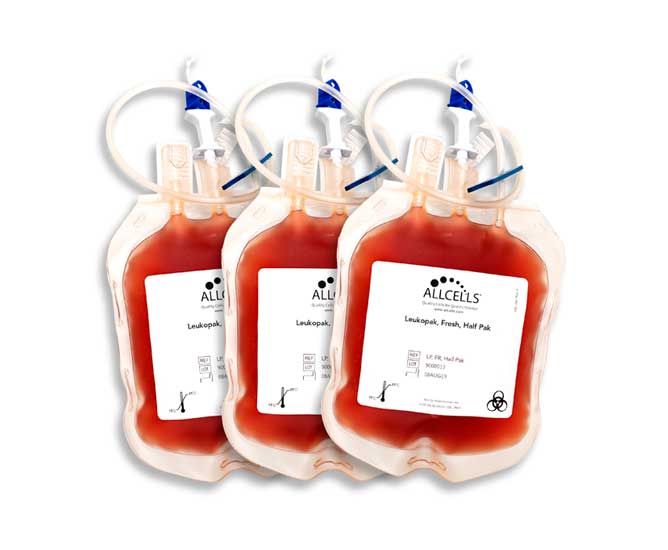
Get the exact number of cells required for your experimental workflow
MADE-TO-ORDER SERVICES

AllCells® Made-To-Order services are designed to meet your specific project needs.
Whole Tissue
Peripheral blood leukopaks are collected from healthy and consenting donors using the Spectra Optia® Apheresis System collection protocol in ACD-A anticoagulant. During collection, autologous plasma is combined with apheresed cells at a 4:6 ratio. Leukopak collections result in large yields of single-donor mononuclear cells (MNCs).
Isolated Cells
We offer a variety of cell populations isolated from Leukopaks including MNCs, T cells, B cells, NK cells and monocytes.
- MNCs are isolated from Leukopaks treated with ammonium chloride to remove red blood cells (RBCs) and washed to remove platelets.
- Isolated T cells, B cells, NK cells and monocytes using positive immunomagnetic bead separation – beads bind to the target cell population from the heterogenous mixture; labeled target cells remain while the non-target cells are removed.
- Isolated T cells, B cells, NK cells and monocytes using negative immunomagnetic bead separation – beads bind to the non-target cell population to deplete them from the mixture leaving behind the unlabeled cells of interest.
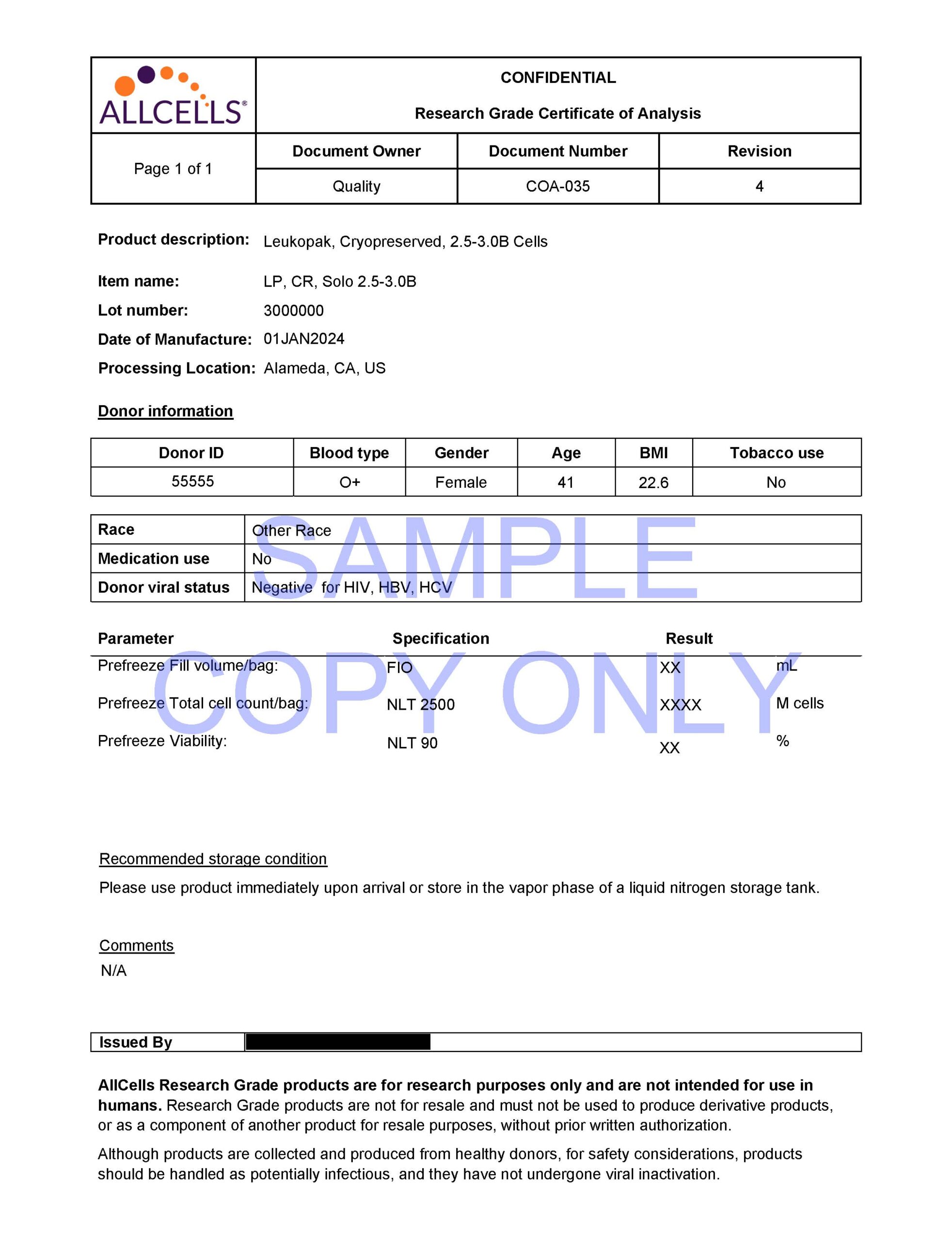
All our products are quality controlled for cell count and viability and are determined using a validated AO/PI protocol.
Research & Development Applications
- Fundamental Research (Proliferation, Biomarker Discovery)
- Drug Discovery
- Immuno-oncology
- Cell Therapy process development
At Discovery, we believe that your success is our success. We believe that sharing our knowledge and expertise is more than a business practice, it’s a responsibility. And we believe that collaboration leads to better outcomes. Below is a collection of relevant links to Leukopak resouces including blog posts, webinars and useful protocols to help you become more efficient and more effective. Have technical questions about Leukopak products? Our Customer Success Team is standing by.
WEBINARS
- Precision Donor Management for Complex CGT Projects
- Two Essential Aspects to Ensuring the Success of Allogeneic Therapies
- Primary Cells 101: A webinar on human and animal-derived primary cells
- Using Flow Cytometry to Analyze Rare Hematopoietic Cell Subpopulations Implicated in Disease
- In vitro human primary cell based screening assays for vaccine adjuvant development
BLOG POSTS
- Progressing Ex Vivo Gene Editing Innovations with High-Quality Leukopaks
- Advancing CAR-NK Therapy: Why Leukopak Quality and Donor Characterization Matter
- FAQ Blog Series: What’s the Difference between Positive and Negative Immunomagnetic Cell Separation?
- Overcoming the Global Biomaterial Supply Chain Challenge for the Development of CAR-T Therapies
- A Decade of Innovation: CAR-T Successes and Latest Developments
- Why You Should Start Clinical Grade Cell Supply Discussions Early in Cell and Gene Therapy Development
