AllCells® Mobilized Leukopak
Mobilized leukopaks are collected from healthy donors with FDA-approved drugs to stimulate the bone marrow to release CD34+ hematopoietic stem cells (HSCs) into the bloodstream. Mobilized peripheral blood, enriched in single-donor CD34+ HSPCs is collected through leukapheresis using the Spectra Optia® Apheresis System’s continuous flow centrifugal technology. The ease of collection and high yield of single-donor CD34+ cells is a major advantage to researchers to gain experimental consistency and scalability.
Discovery is the industry leader in supplying mobilized leukopaks with the shortest lead time on the market. We have mobilization regimens available using G-CSF, plerixafor, or a combination of the two drugs as well as CD34+ cells isolated from mobilized leukopaks.
Mobilized Leukopak Formats, Attributes, and Unit Sizes
Discovery offers fresh mobilized leukopaks and cryopreserved CD34+ cells from a variety of dosing regimens. The product chart below provides detailed information on standard formats and unit sizes.
| Product | Regimen Dosing Details | Fresh | Cryopreserved |
|---|---|---|---|
| mLP Reg F | G-CSF x 5 days | 1 Bag, 2 Bags | — |
| mLP Reg H | Dual Mobilization: G-CSF x 5 days + Plerixafor x 2 days | 1 Bag, 2 Bags | — |
| mLP Reg A | Plerixafor x 1 day (11 hrs between injection and collection, 1 collection) | 1 Bag | — |
| mLP Reg B | Plerixafor x 2 days (11 hrs between injection and collection, 2 collections) | 2 Bags | — |
| Reg F CD34+ | G-CSF x 5 days | — | 0.5M, 1M, 5M, 10M, 25M |
| Reg H CD34+ | Dual Mobilization: G-CSF x 5 days + Plerixafor x 2 days | — | 1M, 5M, 10M, 25M |
| Reg B CD34+ | Plerixafor x 2 days (11 hrs between injection and collection, 2 collections) | — | 1M, 5M, 10M, 25M |
Don’t see what you’re looking for? Our mobilized leukopak products can also be made-to-order (MTO) with custom specifications to meet the specific requirements of your research project. For more information about MTO, please visit our MTO page or reach out to our Customer Success Team. Need immediate help? Chat with a live agent now.
Whole Tissue: Healthy, consenting donors are subcutaneously injected by our qualified clinicians using the following regimen guidelines:
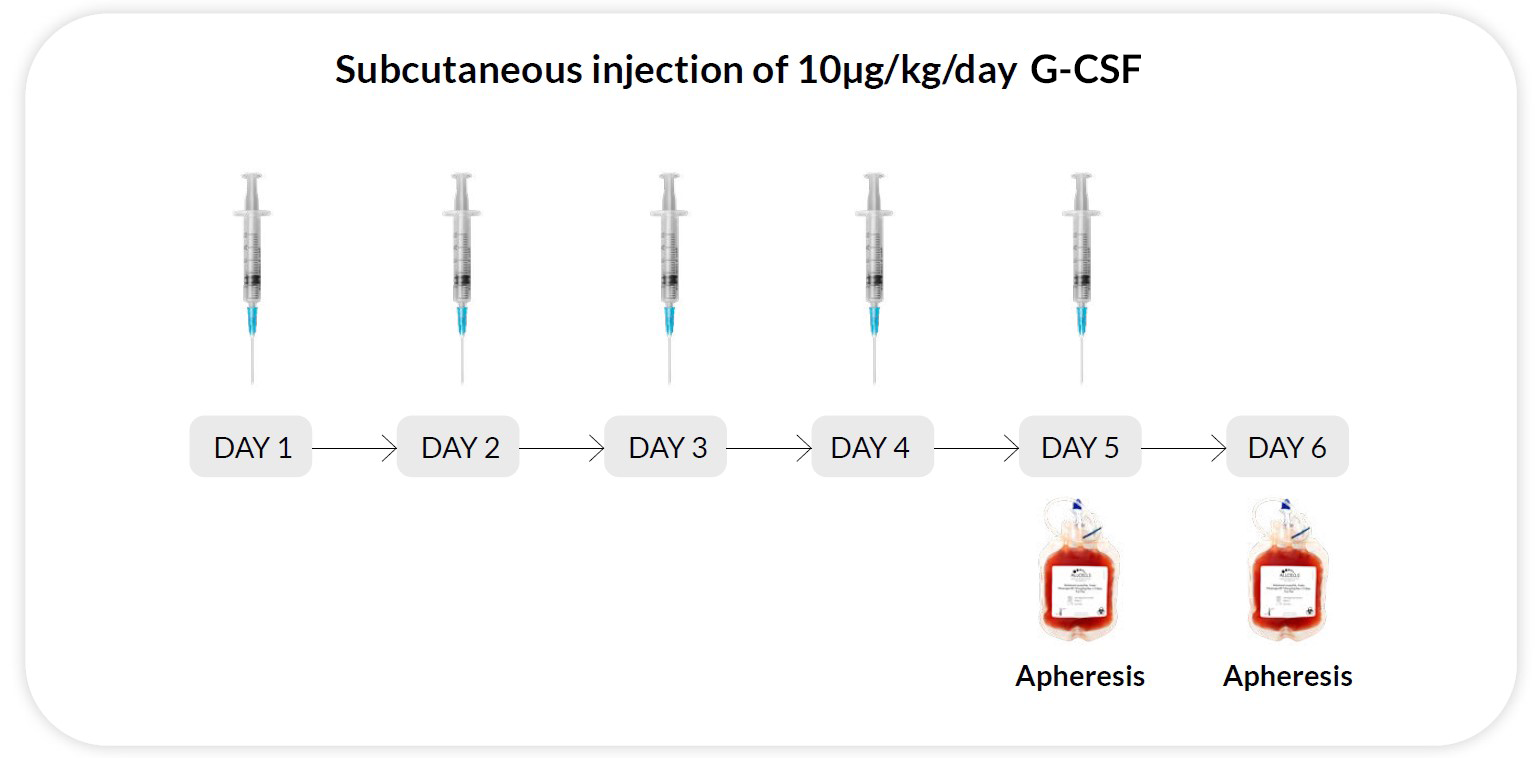
Reg F
G-CSF 10μg/kg/day x 5 days, apheresis on day 5 and 6
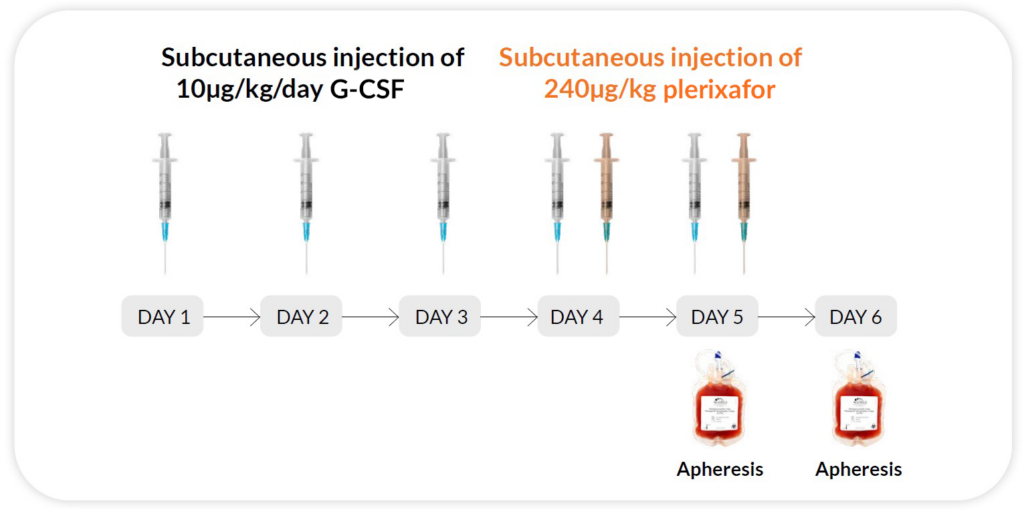
Reg H
G-CSF 10μg/kg/day x 5 days + Plerixafor 240ug/kg/day x 2 days on day 4 and 5 (evenings), apheresis on day 5 and 6
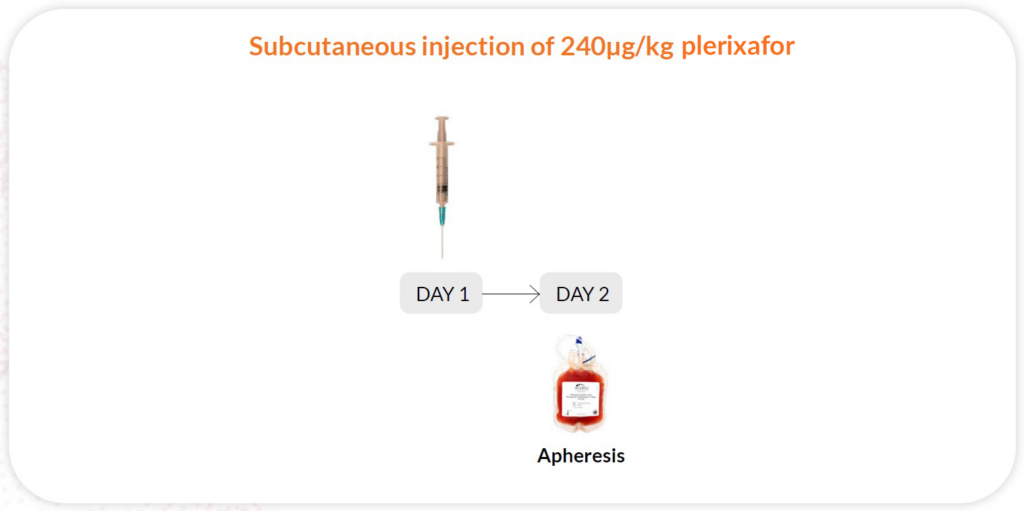
Reg A
Plerixafor 240-μg/kg/day x 1 day, apheresis day 2; 11 hours between injection and collection
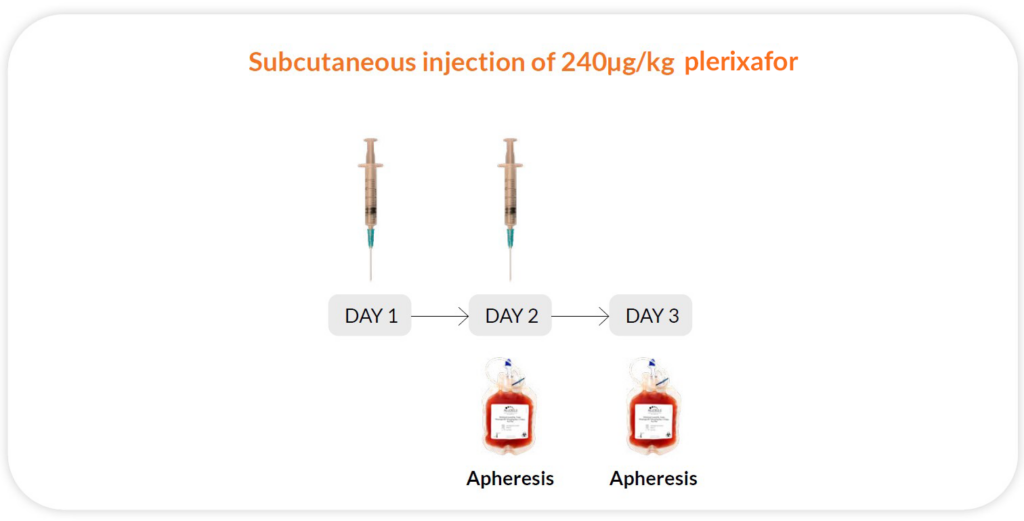
Reg B
Plerixafor 240μg/kg/day x 2 days, apheresis on day 2 and 3; 11 hours between injection and collection
Isolated Cells: CD34+ HSPCs isolated from any of the mobilized leukapheresis collections are available. Positive immunomagnetic separation is used where CD34+ beads bind to target cells in the heterogeneous population. CD34+ cells remain bound to the beads while unwanted cells are removed.
At Discovery, we believe that your success is our success. We believe that sharing our knowledge and expertise is more than a business practice, it’s a responsibility. And we believe that collaboration leads to better outcomes. Below is a collection of relevant links to Mobilized Leukopak resouces including blog posts, webinars and useful protocols to help you become more efficient and more effective. Have technical questions about Mobilized Leukopak products? Our Customer Success Team is standing by.
WEBINARS
BLOG POSTS
- Dual Mobilization Enhances CD34+ Yield Across All Donor Types
- The Therapeutic Potential of HSC-based Gene Therapies
- Why You Should Start Clinical Grade Cell Supply Discussions Early in Cell and Gene Therapy Development
- What You Need to Know About Regenerative Medicine in 2022: Industry and Tech Trends
- A Donor-Centric Approach to Cell and Gene Therapy
- FAQ Series: Comparison of CD34+ Cells from Different Tissue Sources
AllCells® Made-to-Order (MTO) Services
Our state-of-the-art on-site collection and processing facilities enables us to offer custom, MTO products to meet the unique needs of our customers. From donor procurement to product shipment, Discovery’s experienced team of experts will help you define, design, and deliver your customized primary cell material.
We can accommodate specific donor requests, custom mobilization regimen requirements, bulk isolations, cryopreservation needs and more.

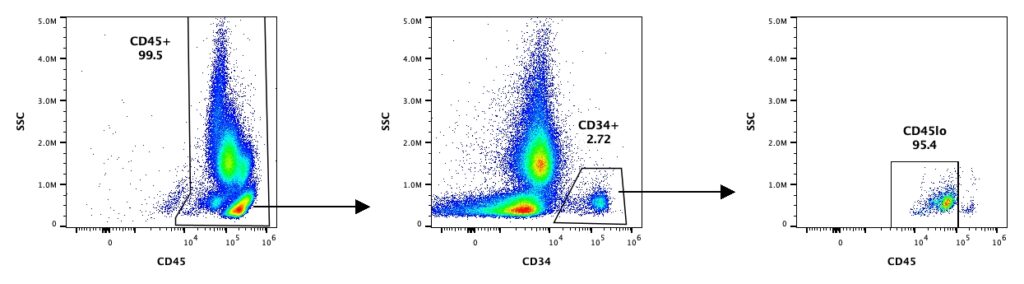
ISHAGE Services
Accurately enumerating CD34+ cells within a heterogeneous cell population is important because this small subset of cells is responsible for multilineage engraftment. The International Society of Hematotherapy and Graft Engineering (ISHAGE) established a standardized, four-parameter flow cytometric method followed by a sequential gating strategy to quantitate CD34+ cells in apheresis products, peripheral blood, and bone marrow used widely in research and clinical settings. Our CD34+ isolated cell products are assessed and quality-controlled using the standardized ISHAGE protocol to quantify viable CD34+ upon request.